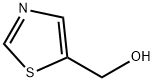
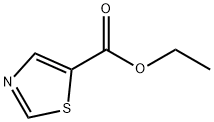
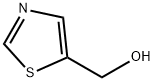
The general procedure for the synthesis of 5-hydroxymethylthiazole from ethyl thiazole-5-carboxylate was as follows: a 250 mL THF solution of lithium aluminum hydride (2.89 g, 76 mmol) was added to a pre-cooled (ice-bath) 500 mL three-neck flask. Subsequently, ethyl thiazole-5-carboxylate (11.82 g, 75.68 mmol) was dissolved in 100 mL of THF and slowly added dropwise to the reaction system over a period of 1.5 h to avoid violent foaming. After the dropwise addition was completed, stirring of the reaction mixture was continued for 1 hour. Upon completion of the reaction, 2.9 mL of water, 2.9 mL of 15% NaOH solution, and 8.7 mL of water were carefully added sequentially to quench the reaction. The solid salt was removed by filtration and the filtrate was retained. The crude product salt was heated at reflux in 100 mL of ethyl acetate for 30 minutes and then filtered. The filtrates from the two filtrations were combined, dried with anhydrous Na2SO4 and subsequently concentrated under reduced pressure. The product was purified by silica gel column chromatography using sequentially 0%, 2%, and 4% chloroform solution of methanol as eluent, and fractions with an Rf value of 0.3 (chloroform solution of 4% methanol) were collected and left to solidify to give the target compound in 75% yield. The structure of the product was confirmed by NMR (CDCl3) δ 4.92 (s, 2H), 7.78 (s, 1H), 8.77 (s, 1H) and mass spectra ((M + H)+ = 116).