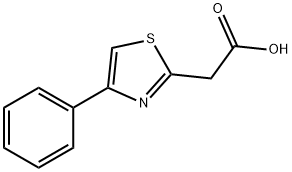
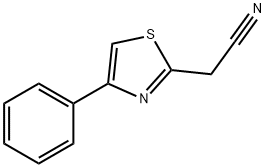
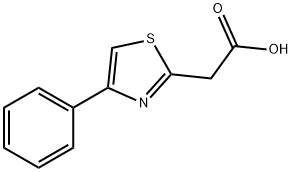
General procedure for the synthesis of 4-phenyl-2-thiazoleacetic acid from 2-(4-phenylthiazol-2-yl)acetonitrile: To a solution of 2-(4-phenylthiazol-2-yl)acetonitrile (130 mg, 0.649 mmol) in ethanol (1.5 mL) was added a 5 M sodium hydroxide solution (0.389 mL, 1.947 mmol). The reaction mixture was heated to reflux for 1 hour. Upon completion of the reaction, the reaction solution was diluted with 1 M aqueous hydrochloric acid solution (5 mL) and subsequently extracted five times with chloroform (10 mL). The organic layers were combined and dried with magnesium sulfate. The solvent was removed by evaporation under reduced pressure. The residue was purified by silica gel column chromatography (eluent ratio chloroform:methanol:acetic acid = 10:1:0.1) to afford 4-phenyl-2-thiazoleacetic acid (77.2 mg, 54% yield) as a brown oil.1H-NMR (DMSO-d6) data were as follows: δ (ppm) 4.13 (s, 2H), 7.30-7.37 (m, 1H). 7.39-7.47 (m, 2H), 7.90-7.97 (m, 2H), 8.04 (s, 1H), 12.81 (brs, 1H).