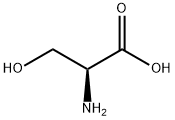
302-84-1
| Name | DL-Serine |
| CAS | 302-84-1 |
| EINECS(EC#) | 206-130-6 |
| Molecular Formula | C3H7NO3 |
| MDL Number | MFCD00064223 |
| Molecular Weight | 105.09 |
| MOL File | 302-84-1.mol |
Chemical Properties
| Appearance | White crystalline powder |
| Melting point | 240 °C (dec.) (lit.) |
| Boiling point | 197.09°C (rough estimate) |
| density | 1.53 |
| refractive index | 1.4368 (estimate) |
| storage temp. | Store at RT. |
| solubility | H2O: soluble |
| form | Crystalline Powder |
| pka | 2.19(at 25℃) |
| color | White |
| Odor | Odorless |
| Water Solubility | 50.23 g/L (25 ºC) |
| Merck | 14,8460 |
| BRN | 1721402 |
| Cosmetics Ingredients Functions | FRAGRANCE HAIR CONDITIONING ANTISTATIC SKIN CONDITIONING |
| InChI | 1S/C3H7NO3/c4-2(1-5)3(6)7/h2,5H,1,4H2,(H,6,7) |
| InChIKey | MTCFGRXMJLQNBG-UHFFFAOYSA-N |
| SMILES | NC(CO)C(O)=O |
| LogP | -3.460 |
Safety Data
| Hazard Codes | Xi |
| Risk Statements | |
| Safety Statements | |
| WGK Germany | 3 |
| RTECS | VT8100000 |
| TSCA | Yes |
| HS Code | 29225000 |
| Storage Class | 11 - Combustible Solids |
| Safety Profile |