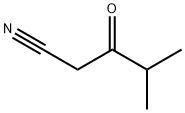
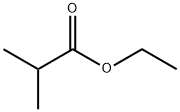
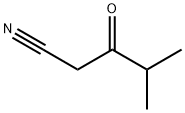
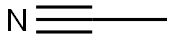GENERAL METHOD: Ethyl isobutyrate (6.65 mmol, 1 eq.) was dissolved in THF (30 mL, industrial grade, 0.2% water) at room temperature with stirring at about 230 rpm for 5 minutes. Subsequently, potassium tert-butoxide (1.57 g, 14.0 mmol, 95%, 2 eq.) was rapidly added to the above THF solution. After sufficiently stirring the reaction vial, acetonitrile (6.65 mmol, 1 eq.) was added. The resulting mixture was continued to be stirred at room temperature. Upon completion of the reaction, the reaction was quenched by the addition of water (50 mL) and stirring was continued for 5 minutes. Next, ethyl acetate (40 mL) and HCl solution (1 mL, 12 M) were added, and the organic layer was separated and dried with anhydrous magnesium sulfate. The solvent was removed by evaporation under reduced pressure, and the residue was loaded onto the top of an open silica gel column (column size: 3 × 15 cm, eluent hexane/ethyl acetate (3:1, v/v); for specific products, the column size was adjusted to 3.5 × 8 cm, and the eluent was CH2Cl2). The fraction containing the target product 3-oxo-4-methylpentanenitrile was collected and evaporated under reduced pressure to give the purified β-ketonitrile product.