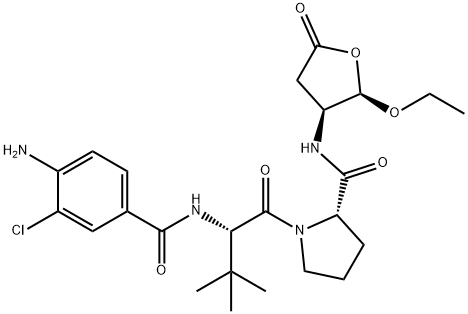
The cell-permeable prodrug of the caspase-1/4-selective inhibitor VRT-43198 (Ki in nM = <0.6/Caspase-4, 0.8/Caspase-1, 100/Caspase-8, 560/Caspase-6, 1,030/Caspase-9, 9,000/Granzyme B, 16,000/Caspase-7, 21,500/Caspase-3; IC50 >100 μM against Cathepsin B & Trypsin). In addition to inhibiting LPS-induced IL-1β production in primary human PBMC cultures (IC50 ~1 μM), VX-765 is also effective in preventing HIV infection-induced IL-1β production and pyroptosis of CD4 T cells in human lymphoid aggregate cultures (HLAC; CD4 population = 29.2% in non-infected control cultures, 8.3% vs. 30.2% in infected cultures with or without 5 μM VX-765). VX-765 is orally available in mice (Blood Cmax = 0.78 μg/mL = 1.53 μM; Tmax = 1.0 h; AUClast = 2.06 μg · h/mL; 84 mg/kg, p.o.) and shown to display in vivo anti-inflammatory efficacy against LPS-induced plasma IL-1β production (EDmax = 100 mg/kg, p.o.), Oxazolone-induced delayed-type hypersensitivity (EDmax = 50 mg/kg, p.o.), collagen-induced arthritis (EDmax = 100 mg/kg, p.o.). When administered via intraperitoneal injection, VX-765 is also demonstrated to suppress the severity of seizure induction (EDmax = 50 mg/kg i.p.) among rats receiving kainic acid via intracerebroventricular injection.