Synthesis
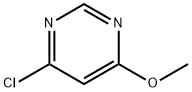
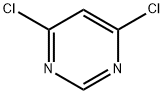
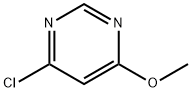
A) Methanol (5.4 mL) was slowly added dropwise to a suspension of tetrahydrofuran (THF, 200 mL) containing 60% sodium hydride (8.05 g) under nitrogen protection, keeping the reaction temperature at 0 °C. Subsequently, a solution of 4,6-dichloropyrimidine (20.0 g) in THF (45 mL) was added to the reaction system at 0 °C, and the reaction continued to be stirred at 0 °C for 1 hour. Upon completion of the reaction, 1N hydrochloric acid was slowly added to the reaction mixture at 0 °C for neutralization, followed by extraction of the reaction product with ethyl acetate. The organic phase was washed sequentially with water and saturated brine and dried with anhydrous magnesium sulfate. The solvent was removed by concentration under reduced pressure and the residue was recrystallized by hexane to afford the target product 4-chloro-6-methoxypyrimidine (10.2 g) as light yellow crystals. The product was characterized by 1H NMR (300 MHz, DMSO-d6): δ 3.96 (3H, s), 7.19 (1H, s), 8.69 (1H, s).
References
[1] Tetrahedron Letters, 2005, vol. 46, # 23, p. 3977 - 3979
[2] European Journal of Medicinal Chemistry, 2005, vol. 40, # 9, p. 862 - 874
[3] Patent: US2013/143870, 2013, A1. Location in patent: Paragraph 0267; 0268; 0269
[4] Patent: US2013/137688, 2013, A1. Location in patent: Paragraph 0221; 0222; 0223; 0224
[5] Patent: US2013/158038, 2013, A1. Location in patent: Paragraph 0134; 0135