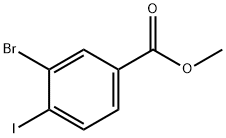
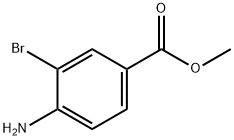
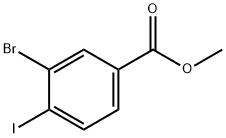
The general procedure for the synthesis of methyl 3-bromo-4-iodobenzoate from methyl 4-amino-3-bromobenzoate was as follows: methyl 4-amino-3-bromobenzoate (5.0 g, 22.0 mmol, purchased from Aldrich) was dissolved in acetone (35 mL), followed by the addition of 6 M hydrochloric acid (35 mL). The reaction mixture was cooled to 0 °C and sodium nitrite (NaNO2, 1.84 g, 26.1 mmol) dissolved in 10 mL of water was added dropwise. After stirring the reaction mixture at 0 °C for 2 h, potassium iodide (KI, 5.47 g, 32.6 mmol) dissolved in 20 mL of water was added slowly. The reaction mixture was gradually warmed to room temperature and stirring was continued for 1 hour. Upon completion of the reaction, the reaction mixture was diluted with water and extracted with ethyl acetate (EtOAc, 2 x 150 mL). The organic phases were combined and dried over anhydrous sodium sulfate (Na2SO4) and subsequently concentrated under vacuum. The residue was purified by column chromatography (using an 80 g Redi-Sep column) with the eluent gradually adjusted from 100% heptane to 20:80 ethyl acetate:heptane, resulting in the target product methyl 3-bromo-4-iodobenzoate (4.1 g, 55% yield) as a solid. The 1H NMR (CDCl3) data of the product were as follows: δ 8.27 (1H), 7.98 (1H), 7.64 (1H), 3.94 (3H).