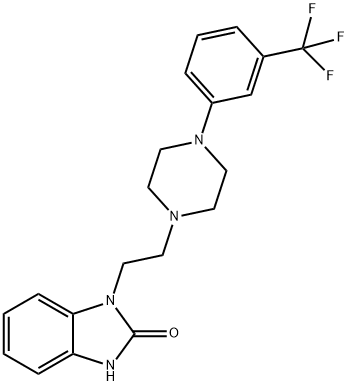
Definition
ChEBI: An N-alkylpiperazine that is 1-[2-(1,3-dihydro-2-oxobenzimidazol-1-yl)ethyl]piperazine in which the remaining amino proton is replaced by a 3-(trifluoromethyl)phenyl group. A multifunctional serotonin agonist and antagonist which is used
for the treatment of pre-menopausal women with hypoactive sexual desire disorder.
Description
Flibanserin is a drug originally
developed by Boehringer-Ingelheim and later Sprout Pharmaceuticals, which was approved in 2015 by the FDA for the
treatment of premenopausal women with hypoactive sexual
desire disorder (HSDD). The drug, which was originally
developed for the treatment of depression by Boehringer-
Ingelheim, is a full agonist of the 5-HT1A receptor, an
antagonist of the 5-HT2A receptor, and a partial agonist of
the dopamine-4 (D4) receptor, which triggers increased
dopamine and norepinephrine levels along with decreased
serotonin levels. In three randomized trials
involving 2400 premenopausal women, the drug was found
to increase the number of satisfying sexual events by 0.5-1.0
events per month and increased sexual desire on average by
10-12% over placebo. Side effects include decreased blood
pressure and loss of consciousness, especially in subjects who
consumed alcohol.
Biochem/physiol Actions
Flibanserin is a 5-HT1A receptor agonist and 5-HT2A receptor antagonist. Flibanserin has high affinity for human 5-HT1A receptors (Ki = 1 nm) and lower affinity for 5-HT2A (Ki = 49 nm) and D4 (Ki = 4–24 nm) receptors, and negligible affinity for a variety of other neurotransmitter receptors and ion channels. Flibanserin was investigated as a novel, non-hormonal treatment for pre-menopausal women with Hypoactive Sexual Desire Disorder (HSDD). Flibanserin. Recently, Flibanserin was found to reduce L-DOPA-induced dyskinesia in a model of Parkinson′s Disease.
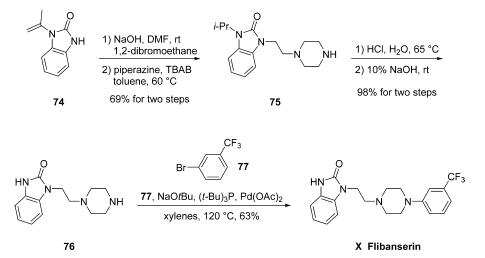
Synthesis
The large-scale synthesis of flibanserin (X) mostly follows a
patent from Symed Laboratories Limited which demonstrated
hundred-gram-scale preparation of the drug as described in
Scheme. Starting from commercially available 1-(prop-1-
en-2-yl)-1,3-dihydro-2H-benzo[d]imidazol-2-one (74), installation
of an ethylene side chain was accomplished under
conventional alkylation conditions with 1,2-dibromoethane
and base, and this event was immediately followed by a second
alkylation reaction involving piperazine to secure piperazinyl
benzimidazolone 75. Interestingly, the enamine double bond within 74 was apparently reduced to the corresponding
isopropyl group under these conditions. Although the authors
do not comment about this reduction directly, similar examples
of olefin reduction under non-hydrogenative alkylation
conditions have been reported in the literature separately by
both Pai and Ryu. Removal of the isopropyl group was
facilitated by means of aqueous sodium hydroxide to afford 76,
which underwent N-arylation under Buchwald conditions with
1-bromo-3-(trifluoromethyl)benzene 77 to furnish flibanserin
(X) in 63% yield.
in vitro
previous study found that flibanserin was a 5-ht(1a) agonist, a very weak partial agonist on dopamine d(4) receptors, and a 5-ht(2a) antagonist. flibanserin could reduce neuronal firing rate and flibanserin-induced reduction in firing rate was likely mediated via stimulation of postsynaptic 5-ht(1a) receptors. moreover, flibanserin could quickly desensitize somatic 5-ht autoreceptors and enhance tonic activation of postsynaptic 5-ht(1a) receptors. in addition, flibanserin was able to reduce synthesis and extracellular levels of 5-ht in the cortex [1].
in vivo
previous animal study showd that flibanserin had antidepressant-like activity in most animal models sensitive to antidepressants, and such activity seemed different from that exerted by other antidepressants. in addition, flibanserin did not show consistent effects in animal models of anxiety and seemed to exert potential antipsychotic effects [1].
References
[1] borsini f, evans k, jason k, rohde f, alexander b, pollentier s. pharmacology of flibanserin. cns drug rev. 2002 summer;8(2):117-42.
[2] katz m et al. efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the begonia trial. j sex med. 2013 jul;10(7):1807-15.