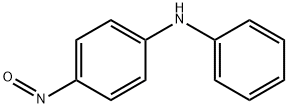
General Description
Green plates with bluish luster or a black powder.
Reactivity Profile
P-NITROSODIPHENYLAMINE(156-10-5) neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides. May react with strong oxidizing agents .
Air & Water Reactions
Insoluble in water.
Hazard
Questionable carcinogen.
Potential Exposure
Used as a chemical intermediate for
dyes and pharmaceuticals; in making monomers; and vulcanizing
rubber.
Fire Hazard
Flash point data for this chemical are not available; however, P-NITROSODIPHENYLAMINE is probably combustible.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions,including resuscitation mask) if breathing has stopped and
CPR if heart action has stopped. Transfer promptly to a
medical facility. When this chemical has been swallowed,
get medical attention. Give large quantities of water and
induce vomiting. Do not make an unconscious person
vomit.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. UN3077 Environmentally hazardous
substances, solid, n.o.s., Hazard Class: 9; Labels:
9-Miscellaneous hazardous material, Technical Name
Required.
Incompatibilities
Nitrated organics range from slight to
strong oxidizing agents. If mixed with reducing agents,
including hydrides, sulfides and nitrides, they may begin a
vigorous reaction. Reaction with aliphatic amines can
release carcinogenic nitrosamines. Incompatible with
oxidizers (chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Keep away from alkaline
materials, strong bases, strong acids, oxoacids, epoxides.
4-nitrosodiphenylamine neutralizes acids in exothermic
reactions to form salts plus water. May be incompatible
with isocyanates, halogenated organics, peroxides, phenols
(acidic), epoxides, anhydrides, and acid halides. May generate
hydrogen, a flammable gas, in combination with
strong reducing agents such as hydrideds and active metals.
May react with strong oxidizing agents.
Chemical Properties
dark blue to black powder
Chemical Properties
TKB is a black powder or a green plate-like
material with a bluish luster.
Uses
4-?Nitrosodiphenylamine is used in organic synthesis such as in the preparation of 1-(Phenylamino)pseudo-mauveine water soluble dye. Potential mutagenic compound.Environmental toxin on US EPA Toxic Release Inventory list (TRI) list.
Uses
Has been used as retardant in vulcanizing rubber.
Definition
ChEBI: Para-Nitrosodiphenylamine is a member of benzenes.
Purification Methods
The amine forms dark green crystals from EtOH or *C6H6 (m 143o). It has UV at max 421nm (EtOH) and is used for detecting Pd and Rh. It is highly toxic and a possible carcinogen.[Beilstein 12 H 207, 12 II 122, 12 III 347, 12 IV 1860..]