Synthesis
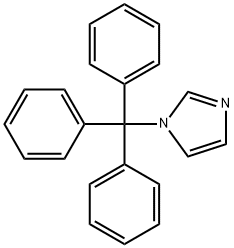
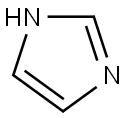
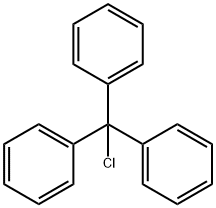
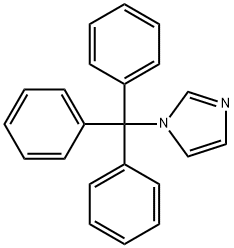
The reaction was carried out in a 50 L autoclave using imidazole (880 g, 12.9 mol, 1.0 eq.) and triphenylchloromethane (3605.0 g, 12.9 mol, 1.0 eq.) as raw materials. First, imidazole was dissolved in 12 L of DMF and cooled to below 0 °C. Subsequently, triethylamine (1308 g, 12.9 mol, 1.0 eq.) was added and the reaction was kept for 30 min. A DMF solution of triphenylchloromethane (16 L) was slowly added dropwise to the reaction mixture at 0°C. After the dropwise addition was completed, the reaction system was warmed to 15°C and the reaction was continued with stirring for 16 hours. Upon completion of the reaction, the reaction mixture was poured into water and a large amount of solid was precipitated. The solid was collected by filtration and the filter cake was washed with water, drained and dried to afford N-trityl imidazole (Compound XIV-1) as a white solid 3835.1 g in 95.6% yield.
References
[1] Patent: CN104086553, 2016, B. Location in patent: Paragraph 0065-0068
[2] Patent: US2010/16610, 2010, A1. Location in patent: Page/Page column 28
[3] Journal of Heterocyclic Chemistry, 1982, vol. 19, p. 253 - 256
[4] Tetrahedron, 1999, vol. 55, # 13, p. 4109 - 4122
[5] Patent: US6476216, 2002, B1