Synthesis
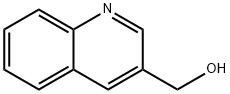
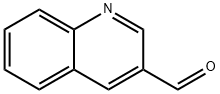
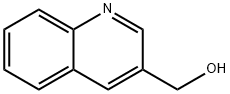
Step 1: Sodium borohydride (240 mg, 6.4 mmol) was added batchwise to a solution of quinoline-3-carbaldehyde (910 mg, 5.8 mmol) in methanol (10 mL) at room temperature. The reaction mixture was stirred for 3 hours and then the reaction was quenched with saturated aqueous ammonium chloride solution (10 mL). Subsequently, the reaction mixture was extracted with ethyl acetate (3 x 30 mL). The organic phases were combined, washed with brine and dried with anhydrous magnesium sulfate. After filtration, the filtrate was concentrated under reduced pressure to afford the crude quinolin-3-ylmethanol (795 mg, 86% yield).
References
[1] Journal of Organic Chemistry, 1990, vol. 55, # 19, p. 5344 - 5347
[2] European Journal of Organic Chemistry, 2009, # 26, p. 4458 - 4467
[3] Patent: WO2008/115516, 2008, A2. Location in patent: Page/Page column 65
[4] Patent: WO2005/123724, 2005, A1. Location in patent: Page/Page column 73
[5] Heterocycles, 1999, vol. 51, # 8, p. 1883 - 1889