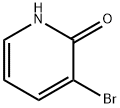
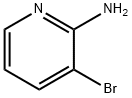
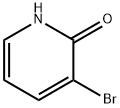
The general procedure for the synthesis of 3-bromo-2-hydroxypyridine from 2-amino-3-bromopyridine was as follows: sodium nitrite (24.2 g, 0.35 mol, 4.84 eq.) was dissolved in water (175 mL) at 0 °C and slowly added dropwise to a solution containing 2-amino-3-bromopyridine (12.5 g, 72.4 mmol) and sulfuric acid (35 mL, 0.66 mol , 9.10 eq.) in a solution of water (175 mL). The reaction mixture was stirred at 0 °C for 1 hour. Subsequently, the reaction mixture was neutralized with sodium hydroxide solution to pH=7. The neutralized solution was extracted with chloroform (3 x 200 mL), the organic phases were combined and washed with brine (200 mL), dried over magnesium sulfate, filtered and concentrated in vacuum to afford the milky white solid product 3-bromo-2-hydroxypyridine (11.3 g, 90% yield). The product was characterized as follows: melting point 183 °C; IR (pure) νmax 3339, 3105, 2991, 2942, 1776, 1650, 1610, 1464 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.87 (dd, J = 7.3, 1.9 Hz, 1H), 7.49 (dd, J = 6.4, 1.9 Hz, 1H), 6.24 (dd, J = 7.3, 6.4 Hz, 1H) ppm; 13C NMR (125 MHz, CDCl3) δ 161.9, 143.9, 134.4, 115.7, 107.7 ppm; HRMS (ESI) m/z [M + Na]+ Calculated value: 195.9369, measured value: 195.9373 (deviation +2.07 ppm).