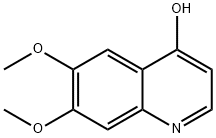
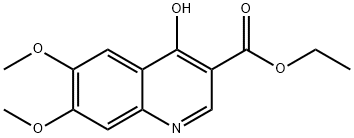
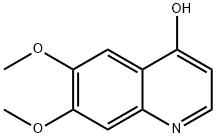
General procedure for the synthesis of 4-hydroxy-6,7-dimethoxyquinoline-3-carboxylic acid from ethyl 4-hydroxy-6,7-dimethoxyquinoline-3-carboxylate: ethyl 4-hydroxy-6,7-dimethoxyquinoline-3-carboxylate (700 mg, 2.53 mmol) was added to potassium hydroxide (450 mg, 7.6 mmol) dissolved in 20 mL of H2O/EtOH (1:1, v /v) solution. The mixture was placed in a sealed container (XP-500 Plus container) and heated using microwaves (MARS 5 Microwave System) at 180°C, 260-280 psi pressure for 50 min. Upon completion of the reaction, the mixture was cooled to room temperature and transferred to a flask. Subsequently, the solution was acidified to pH about 6 with acetic acid (about 2 mL), saturated with sodium chloride, and extracted with tetrahydrofuran (3 x 100 mL). The organic layers were combined, washed with brine and concentrated to give 6,7-dimethoxyquinolin-4-ol in 90% yield.1H NMR (400 MHz, DMSO-d6) δ ppm: 3.81 (s, 3H), 3.84 (s, 3H), 5.93 (d, J=7.3 Hz, 1H), 7.05 (s, 1H), 7.42 (s, 1H), and 7.76 (d, J=7.3 Hz, 1H).