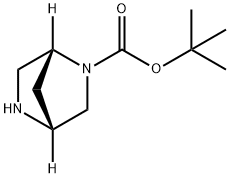
Example 15B: Synthesis of tert-butyl (1R,4R)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate
The product of Example 15A, (1R,4R)-5-benzyl-2,5-diazabicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester (2 g, 6.9 mmol) was dissolved in 50 mL of ethanol and 10% Pd/C catalyst (150 mg) was added. The reaction was stirred under hydrogen atmosphere (1 atm) for 16 hours. After completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated under reduced pressure to give the white solid product tert-butyl (1R,4R)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate (1.28 g, 93.4% yield).
Product characterization data:
1H NMR (DMSO-d6, MHz) δ 1.39 (s, 9H), 1.54 (d, J = 5.6 Hz, 1H), 1.58 (t, J = 9.5 Hz, 1H), 2.70-2.81 (m, 2H), 3.17 (m, 1H), 3.50 (s, 1H), 4.17 (d, J = 10.17 Hz, 1H).
MS (DCI/NH3) m/z 199 (M + H)+, 216 (M + NH4)+.

![(1R,4R)-tert-butyl 5-benzyl-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate](/CAS/20150408/GIF/134003-83-1.gif)
![tert-butyl 2,5-diazabicyclo[2.2.1]heptane-2-carboxylate hydrochloride](/CAS/GIF/134003-84-2.gif)