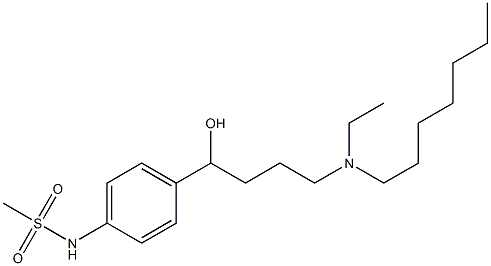
Chemical Properties
Ibutilide Fumarate: C20H36N2O3S?C4H4O4 [122647-32-9]. Crystallized from acetone, melting point 117-119°C. UV absorption maximum (95% ethanol): 228, 267 nm (ε 16670, 894).
Originator
Corvert,Pharmacia and Upjohn,USA
Uses
Cardiac depressant
(anti-arrhythmic).
Definition
ChEBI: Ibutilide is an organic amino compound and a member of benzenes.
Manufacturing Process
A mechanically stirred solution of aniline (139.7 g, 1.5 mole) in pyridine (2 L),
under N2 is cooled in an ice bath. Methanesulfonyl chloride (171.8 g, 1.5
mole) is added dropwise to this solution while the temperature is maintained
at 15°-20°C, which results in a red-orange color change in the reaction
mixture. After the addition is complete the ice bath is removed and the
reaction is allowed to continue at room temperature. The reaction is complete
after 2.5 h. The reaction mixture is concentrated in vacuo and the residue is
combined with 700 ml of water which results in crystallization of a dark red
material.
This material is filtered and washed several times with water. The filtered
material is dissolved in CH2Cl2, washed with brine, dried (Na2SO4), and
concentrated in vacuo. The residue is dissolved in hot ethyl acetate, treated
with Darco (decolorizing carbon) and crystallized to yield methanesulfonanilide
which had a melting point: 93°-94°C.
A mechanically stirred suspension of aluminum chloride (88.0 g, 0.66 moles)
and 150 ml of carbon disulfide under N2 is cooled in an ice bath.
Methanesulfonanilide (30.0 g, 0.175 mol) and succinic anhydride (17.5 g,
0.175 mol) are combined and added rapidly to the cooled reaction mixture.
The ice bath is removed and the mixture is stirred at room temperature for 6
h. The reaction mixture is then heated to 55°C and allowed to continue for 18
h. The reaction mixture is separated into two layers the bottom of which
solidifies.
The upper layer is decanted and the remaining solid layer is decomposed with
ice. The resulting suspension is filtered and the solid is washed several times
with methylene chloride and dissolved in a mixture of saturated sodium
bicarbonate (500 ml) and water (500 ml). This solution is acidified (pH 2) with
HCl and the resulting precipitate is collected by filtration, redissolved in
NaHCO3 and reprecipitated with HCl. The solid, 4-[(methylsulfonyl)amino]-γ-
oxobenzenebutanoic acid, is collected by filtration. Melting point 198°-200°C.
A stirred solution of 4-[(methylsulfonyl)amino]-γ-oxobenzenebutanoic acid
(12.0 g, 0.044 mol) in DMF (100 ml) under N2 is cooled in an ice bath to 5°C
and treated with 1-hydroxybenzotriazole (5.94 g, 0.044 mol) and N,N'-
dicyclohexylcarbodiimide (9.08 g, 0.044 mol). After 1 hour, ethylheptylamine
(6.3 g, 0.044 mol) is added, after an additional 30 min the ice bath is
removed and the mixture is kept at room temperature for 18 h.
The reaction mixture is filtered over a Celite filter aid and the filtrate is
concentrated under vacuum. The resulting material is dissolved in CH2Cl2,
washed with dilute HCl, NaHCO3 and concentrated. The residue is
chromatographed over silica gel (1.25 kg) with 5% MeOH : 1% NH4OH :
CH2Cl2. The N-ethyl-N-heptyl-γ-oxo-4-[(methylsulfonyl)amino]benzenebutanamide thus obtained is crystallized from EtOAc to yield 10.77 g,
melting point 100°-102°C.
To a N2 covered suspension of 0.29 g (7.57 mmol) of LiAlH4 in 10 ml of THF
cooled in an ice bath is added a solution of 1.0 g (2.52 mmol) of N-ethyl-N�heptyl-γ-oxo-4-[methylsulfonyl)amino]benzenebutanamide in 10 ml of THF
over 6 min. The ice bath is then removed and the mixture heated at reflux for
27 h and then stirred at room temperature for 2 days. The mixture is cooled
in an ice bath and there is added dropwise 10 ml of aqueous sodium
potassium tartrate followed by EtOAc and H2O to keep the mixture fluid.
The aqueous fraction is extracted once with EtOAc and the combined EtOAc
fractions are washed in turn with H2O and concentrated in vacuo. The residue
is chromatographed on a 200 ml silica gel column (elution with 6% MeOH :
CH2Cl2 containing 0.5% NH4OH) and 9.7 ml fractions were collected and
treated with Et2O and aqueous NaHCO3. The organic layer is concentrated in
vacuo to yield N-[4-[4-(ethylheptylamino)-1-hydroxybutyl]phenyl]
methanesulfonamide.
Preparation of fumarate (WO Patent 01/07417). To dichloromethane solution
of 4-[4-N-[(Ethylheptylamino)-1-hydroxybutyl]phenyl]methanesulfonamide is
added hemimolar quantities of fumaric acid and heated to reflux until a clear
solution was obtained. Upon cooling the fumarate of 4-[4-N-
[(Ethylheptylamino)-1-hydroxybutyl]phenyl]methanesulfonamide was
obtained.
Brand name
Inocor
(Sterling Winthrop).
Therapeutic Function
Antiarrhythmic
General Description
Ibutilide, N-{4-[4-(ethylheptylamino)-1-hydroxybutyl]phenyl}methanesulfonamide (Corvert), aclass III antiarrhythmic belonging to the methanesulfonanilideclass of agents, is indicated for rapid conversion ofatrial fibrillation or atrial flutter to normal sinus rhythm.Unlike dofetilide, it is not highly specific for the delayedrectifier potassium currents (Ikr) and does have some affinityfor sodium channels.
Clinical Use
Ibutilide (Corvert) is another methanesulfonanilide derivative , but unlike sotalol, it lacks
any β-adrenergic blocking activity. Like sotalol, it exhibits electrophysiologic effects characteristic of
Class III. Ibutilide is used only by intravenous infusion as its fumarate salt.