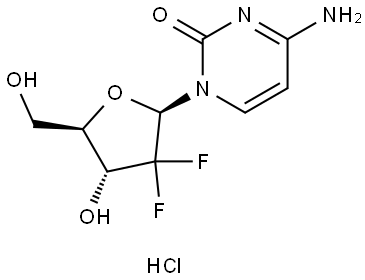
Description
Gemcitabine is a novel nucleoside analog that was launched in 1995 in the
Netherlands for the treatment of non-small cell lung cancer (nsclc) and in Sweden for
pancreatic cancer. Gemcitabine is a prodrug which is phosphorylated intracellularly by
deoxycytadine kinase to its active forms, the di- and triphosphates which bind to DNA
competitively. This insertion inhibits processes required for DNA synthesis and
metabolism, the essential function for both cell replication and repair. Furthermore,
gemcitabine displays an extraordinary array of self-potentiating mechanisms that
increase the concentration and prolong the retention of its active nucleotides in tumor
cells. The title compound has shown activity against a wide spectrum of human solid
tumors including colon, mammary, breast, bladder cancers. Synergistic activity of
gemcitabine with other anticancer agents such as cisplatin has been reported.
Chemical Properties
White crystalline granular, odorless
Originator
Lilly (U.S.A.)
Definition
ChEBI: A 2'-deoxycytidine hydrochloriode having geminal fluoro substituents in the 2'-position. An inhibitor of ribonucleotide reductase, gemcitabine hydrochloride is used in the treatment of various carcinomas, including non-small cell lung cancer, pancreatic ca
cer, bladder cancer and breast cancer.
Brand name
Gemzar (Lilly).
Biological Activity
Deoxycytidine analog that inhibits DNA synthesis. Metabolized to form gemcitabine triphosphate (dFdCTP) and gemcitabine diphosphate (dFdCDP). dFdCTD inhibits ribonucleotide reductase causing a reduction in cellular nucleotides. dFdCTP is incorporated in DNA resulting in DNA strand termination. Displays antitumor activity in vitro and in vivo .
Biochem/physiol Actions
Gemcitabine is a widely used antitumor agents in both clinics and research labs. It is an antineoplastic agent and antimetabolite.
Veterinary Drugs and Treatments
Very limited clinical use and research performed with this drug to
date have demonstrated limited clinical efficacy. However, it potentially
may be useful as a radiosensitizer for non-resectable tumors,
as part of combination protocols, or as a single agent for tumors
not amenable to more accepted therapies. Follow research reports
for the most up-to-date information.
In humans, gemcitabine has shown some efficacy in treating
pancreatic carcinoma, small-cell lung carcinoma, lymphoma, bladder
and other soft tissue carcinomas.
References
1) Mini et al. (2006), Cellular Pharmacology of Gemcitabine; Ann. Oncol. 17 v7
2) Heinemann et al. (1995), Gemcitabine: a modulator of intracellular nucleotide and deoxynucleotide metabolism; Semin. Oncol. 22 11
3) Heinemann et al. (1992), Cellular elimination of 2′,2′-difluorodeoxycytidine 5’triphosphate: a mechanism of self-potentiation; Cancer Res. 52 533
4) Pourquier et al. (2002), Gemcitabine (2′,2′-difluoro-2′-deoxycytidine), an antimetabolite that poisons topoisomerase I; Clin.Cancer Res. 8 2499