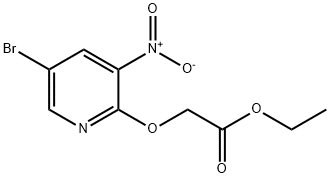
Method I: Under dry conditions, 5-bromo-2-chloro-3-nitropyridine (100 mg, 0.42 mmol) and ethyl ethanoate (0.044 mL, 0.46 mmol) were dissolved in anhydrous THF (5 mL) and the mixture was cooled to 0°C. The reaction was carried out under dry conditions. Sodium hydride (60% dispersed in mineral oil, 0.015 mL, 0.46 mmol) was slowly added under nitrogen protection, followed by gradually warming the reaction mixture to room temperature and stirring for 1 hour. Upon completion of the reaction, the mixture was carefully poured into ice water and extracted with ethyl acetate (2 x 10 mL). The organic layers were combined, washed with saturated saline (10 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by fast column chromatography (silica gel 100-200 mesh) to afford ethyl 2-(5-bromo-3-nitropyridin-2-yloxy)acetate (105 mg, 82% yield). The product was characterized as follows: 1H NMR (300 MHz, DMSO-d6) δ = 1.27 (s, 3H), 4.25 (q, 2H), 5.12 (s, 2H), 8.40 (d, J = 2.07 Hz, 1H), 8.47 (d, J = 2.07 Hz, 1H); ESMS: m/z 306.8 [M + 1].