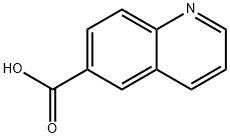
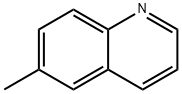
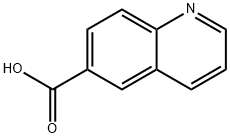
6-Methylquinoline (100.0 mg, 0.70 mmol) was dissolved in a mixed solution of H2O (1.0 mL) and H2SO4 (0.25 mL) at 0 °C and chromium trioxide (272.0 mg, 2.72 mmol) was added in batches. The reaction mixture was refluxed for 24 hours. After completion of the reaction, it was cooled to room temperature and the precipitated crystalline bisulfate precipitate was removed by filtration. The filtrate was dissolved in 10% aqueous sodium hydroxide solution, washed with hexane and the pH was adjusted to acidic with acetic acid to precipitate 6-quinolinecarboxylic acid. The precipitate was collected to give 85.0 mg of 6-quinolinecarboxylic acid in 70% yield. The product could be used in subsequent steps without further purification. The product was characterized as follows: 1H NMR (300 MHz, DMSO-d6) δ 7.61 (dd, 1H, J1 = 8.3 Hz, J2 = 4.2 Hz), 8.08 (d, 1H, J = 8.8 Hz), 8.20 (dd, 1H, J1 = 8.8 Hz, J2 = 1.7 Hz), 8.56 (d, 1H, J = 8.2 Hz), 8.67 (m, 1H, J = 8.2 Hz), 8.67 (m, 1H, J = 8.2 Hz), 8.56 (d, 1H, J = 8.2 Hz), 8.56 (m, 1H, J = 8.2 Hz) 8.67 (m, 1H), 9.00 (dd, 1H, J1 = 4.1 Hz, J2 = 1.5 Hz), 13.20 (br s, 1H); 13C NMR (300 MHz, DMSO-d6) δ 122.9, 127.9, 129.2, 129.5, 130.0, 131.7, 138.2, 150.0, 153.4, 167.4, 167.0, 153.4, 167.4, 167.4, 167.4, 167.4, 167.4, 167.4 153.4, 167.7; ESI-MS m/z 196 [M + Na]+, 174 [M + H]+. Molecular formula: C10H7NO2.