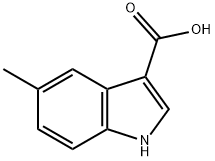
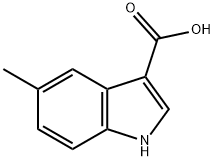
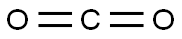Capot Chemical Co., Ltd
Telephone+86 (0) 571 85 58 67 18
Websitehttp://www.capotchem.com/
Shanghai Hanhong Scientific Co.,Ltd.
Telephone021-54306202 13764082696
Websitehttps://www.hanhongsci.com
China Langchem Inc.
Telephone0086-21-58956006
Websitehttps://www.chemicalbook.com/ShowSupplierProductsList19141/0_EN.htm
Hangzhou Yuhao Chemical Technology Co., Ltd
Telephone0571-82693216
Websitehttp://www.chemyuhao.com
Wuhan TCASChem Technology Co., Ltd.
Telephone027-86697669 13986148687
Websitehttps://www.tcaschem.com
Shanghai Topbiochem Technology Co., Ltd
Telephone+86-21-60341587
Websitehttp://www.topbiochem.com
Jiangsu Aikon Biopharmaceutical R&D co.,Ltd.
Telephone025-66113011 17798518460
Websitehttp://www.aikonchem.com/
9ding chemical ( Shanghai) Limited
Telephone4009209199
Websitehttp://www.9dingchem.com
Shanghai Raise Chemical Technology Co.,Ltd
Telephone+86-021-50935922
Websitehttp://www.raise-chem.com
Wuxi Zhangkun Biochemical Technology Co., Ltd.
Telephone+86 0510-85629785
Websitehttps://www.chemicalbook.com/ShowSupplierProductsList16040/0_EN.htm
Shanghai JONLN Reagent Co., Ltd.
Telephone400-0066400 13621662912
Websitehttp://www.jonln.com
Bide Pharmatech Ltd.
Telephone400-400-164-7117 18317119277
Websitehttp://www.bidepharm.com
Wuxi Zhongkun Biochemical Technology Co., Ltd.
Telephone0510-85629785 18013409632
Websitehttp://www.reading-chemicals.com
ShangHai AmK Pharmaceutical Technology Co., Ltd.
Telephone微信 17321281695 18019252918
Websitehttp://www.amkchem.com
Shanghai Jovan Biochemical Technology Co. Ltd.
Telephone+86-021-61654350
Websitehttps://www.chemicalbook.com/ShowSupplierProductsList16421/0_EN.htm
Nanjing Chalf-Pharm Technology Co., Ltd.
Telephone025-57018978 18251899859
Websitehttp://www.chalf-pharm.com
Shanghai Synchem Pharma Co., ltd
Telephone21-619849051-1 18521059765
Websitehttp://www.synchem-pharma.com
JW & Y Pharmlab Co., Ltd.
Telephone021-64340559 13651849907;
Websitehttp://www.jwypharmlab.com.cn