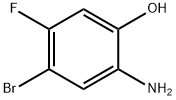
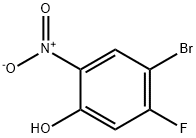
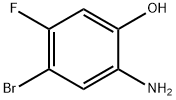
GENERAL STEPS: A suspension of sodium conodisulfite (Na2S2O4, 51.6 g, 296 mmol) in water (122 mL) was slowly added to a refluxed solution of 4-bromo-5-fluoro-2-nitrophenol (14 g, 59.3 mmol) in ethanol (1000 mL). The reaction mixture was continued to reflux for 1 hour. After completion of the reaction, it was cooled to room temperature and filtered to remove the resulting salt. The filtrate was partially concentrated and subsequently saturated aqueous sodium chloride solution was added and extracted with ether. The organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to afford the crude 2-amino-4-bromo-5-fluorophenol (9.2 g, 75% yield), which was used in the subsequent reaction without further purification.1H-NMR (400 MHz, DMSO-d6) δ: 4.65 (br s, 2H, NH2), 6.60 (d, J = 11.3 Hz, 1H ArH), 6.76 (d, J = 7.8 Hz, 1H, ArH), 9.80 (br s, 1H, OH).