What is 1,2,4,5-Tetrakis-(4-formylphenyl)benzene?
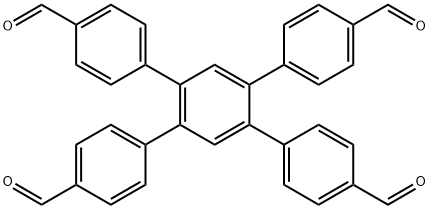
1,2,4,5-Tetrakis-(4-formylphenyl)benzene is an important organic intermediate to synthetize substituted covalent organic frameworks (COFs), in which molecular building blocks are precisely organized into two-dimensional (2D) layered structures or three-dimensional (3D) networks, which have emerged as a novel class of crystalline organic polymers with inherent porosity and structural periodicity. Owing to the tunable structures and high porosity, COFs have gained great interest and shown promising applications in molecule storage and separation, heterogeneous catalysis, sensing, energy storage, and optoelectronics.
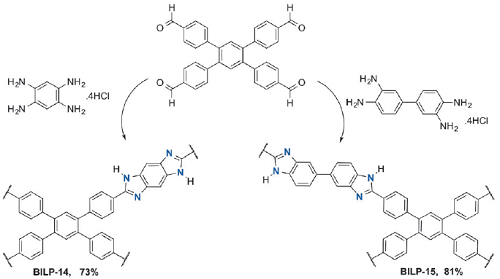
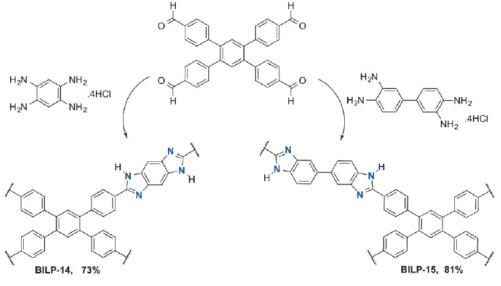
Altarawneh et al. reported [1] its application on the synthesis of two new BILPs containing benzo-bis(imidazole) and 3,3’-bibenzimidazole moieties. It was found that the two BILPs offer multiple interaction sites for CO2 and these sites, along with their binding affinities, can be effectively and conveniently predicted using DFT calculations. The released CO2 from coal-fired plants accounts for about 33–40% of global CO2 emissions, hence efficient CO2 capture from flue gas are at the heart of many academic and industrial research enterprises.
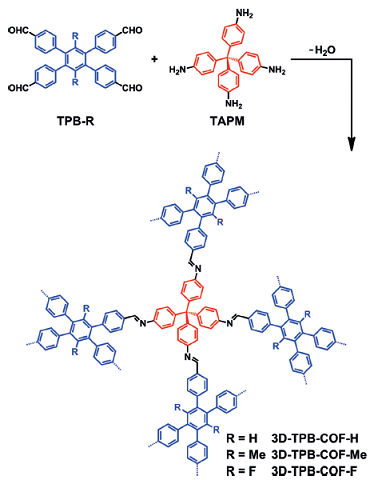
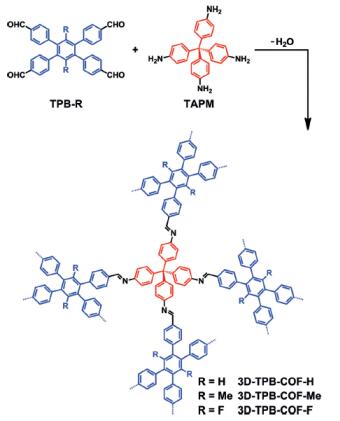
Gao et al. reported [2] its application on the synthesis of three isostructural three-dimensional covalent organic frameworks (3D COFs) with -H, -Me, or -F substituents, which have similar crystallinity and topology. The pore environments were precisely tuned, and the highest selectivity for CO2 over N2 was achieved with polar C-F functional groups. With state-of the- art cRED techniques, the electron diffraction resolution reached up to 0.9–1.0 A, which enabled the localization of all non-hydrogen atoms directly from a polycrystalline COF sample for the first time. The three 3D-TPB-COFs were shown to adopt the same fivefold interpenetrated pts topologies, with the different functional groups being clearly visible.
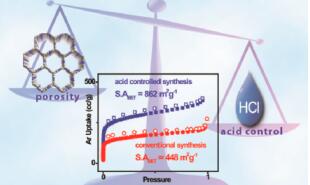
Altarawneh et al. reported [3] its application on the synthesis of benzimidazole-linked polymers (BILPs). The BILPs can be used in selective CO2 capture from gas mixtures containing N2 and CH4 as in the case of flue gas and natural gas, respectively. The BILPs are very promising because of their high porosity, physiochemical stability, and ability to selectivity bind CO2 over other gases which is mainly due to basic N-functionality within the framework that provides interaction sites for CO2.
References
1. Altarawneh S, Behera S, Jena P, El-Kaderi HM. New insights into carbon dioxide interactions with benzimidazole-linked polymers[J]. Chem. Commun., 2014, 50, 3571--3574
2. Gao C. et al. Isostructural Three-Dimensional Covalent Organic Frameworks[J]. Angew. Chem. Int. Ed. 2019, 58:9770–9775
3. Altarawneh S. et al. Effect of Acid-Catalyzed Formation Rates of Benzimidazole-Linked Polymers on Porosity and Selective CO2 Capture from Gas Mixtures[J]. Environ. Sci. Technol. 2015, 49:4715−4723.