What is Pentachlorophenol?
Pentachlorophenol (PCP) is an organochlorine compound used as a pesticide and a disinfectant. First produced in the 1930s, it is marketed under many trade names. It can be found as pure PCP, or as the sodium salt of PCP, the latter which dissolves easily in water. It can be biodegraded by some bacteria, including Sphingobium chlorophenolicum.
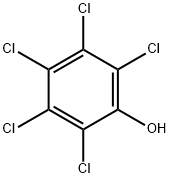
Fig 1. Chemical structure formula and three-dimensional structure of Pentachlorophenol
PCP has been used as a herbicide, insecticide, fungicide, algaecide, and disinfectant and as an ingredient in antifouling paint. Some applications were in agricultural seeds (for nonfood uses), leather, masonry, wood preservation, cooling-tower water, rope, and paper. Its use has declined due to its high toxicity and slow biodegradation.Two general methods are used for preserving wood. The pressure process method involves placing wood in a pressure-treating vessel, where it is immersed in PCP and then subjected to applied pressure. In the nonpressure process method, PCP is applied by spraying, brushing, dipping, or soaking[1].
People may be exposed to PCP in occupational settings through the inhalation of contaminated workplace air and dermal contact with wood products treated with PCP. Also, general population exposure may occur through contact with contaminated environment media, particularly in the vicinity of wood-treatment facilities and hazardous-waste sites. In addition, some other important routes of exposure seem to be the inhalation of contaminated air, ingestion of contaminated ground water used as a source of drinking water, ingestion of contaminated food, and dermal contact with soils or products treated with the chemical.Pentachlorophenol may be measured in plasma or urine as an index of excessive exposure. This is usually performed by gas chromatography with electron-capture or mass-spectrometric detection. Since urine contains predominantly conjugated PCP in chronic exposure situations, prior hydrolysis of specimens is recommended. The current ACGIH biological exposure limits for occupational exposure to PCP are 5 mg/l in an end-of-shift plasma specimen and 2 mg/g creatinine in an end-of-shift urine specimen.
PCP is quickly absorbed through the gastrointestinal tract following ingestion. Accumulation is not common, but if it does occur, the major sites are the liver, kidneys, plasma protein, spleen, and fat. Unless kidney and liver functions are impaired, PCP is quickly eliminated from tissues and blood, and is excreted, mainly unchanged or in conjugated form, via the urine. Single doses of PCP have half-lives in blood of 30 to 50 hours in humans. Biomagnification of PCP in the food chain is not thought to be significant due to the fairly rapid metabolism of the compound by exposed organisms.
PCP can be produced by the chlorination of phenol in the presence of catalyst (anhydrous aluminium or ferric chloride) and a temperature up to about 191°C. This process does not result in complete chlorination and commercial PCP is only 84-90% pure. The main contaminants include other polychlorinated phenols, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans. Some of these species are even more toxic than the PCP itself[2-3].
References
[1] Fiege, H.; Voges, H.-M.; Hamamoto, T; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
[2] Edgerton TR, Moseman RF. Determination of pentachlorophenol in urine: the importance of hydrolysis. J. Agr. Food Chem. 27: 197-199, 1979.
[3] R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, California, 2008, pp. 1197-1200.
[4] https://pubchem.ncbi.nlm.nih.gov/compound/992
[5] http://www.chemspider.com/Chemical-Structure.967.html?rid=f13eb049-ca84-4674-b9b6-5bad4b6f7bab