What is Methyl Perfluorobutyl Ether?
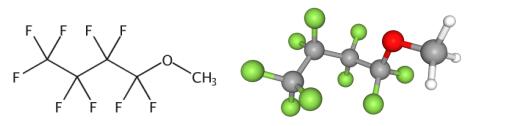
Methyl perfluorobutyl ether (1,1,1,2,2,3,3,4,4-nonafluoro-4-methoxybutane; 1,1,1,2,2,3,3,4,4-Nonafluoro-4-methoxybutane; (perfluorobutoxy)methane; 1-methoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane; 1-methoxynonafluorobutane; CF 61; cosmetic fluid CF 61; GransilSiW 7100; HFC 7100; HFE 449; HFE 449mccc; methoxyperfluorobutane; methyl 1,1,2,2,3,3,4,4,4-nonafluorobutyl ether; methyl nonafluorobutyl ether; methyl perfluorobutyl ether; nonafluorobutyl methyl ether; Novec TM 7100; perfluorobutyl methyl ether; n7100;) [1] is a clear, colorless, low-odor, non-toxic, non-corrosive, non-flammable liquid substance with a chemical formula of C5H3F9O and a molecular weight of 250.06 [2]. Since it has low flammability, relatively low vapor pressure, low heat of vaporization, and low surface tension, it is considered to replace the chlorine-containing cleaning solvents such as 1,1,1-trichloroethane, CFC-113 (CF2ClCFCl2), and HCFC-141b (CH3CFCl2). Besides, it is a fluorinated ether solvent that is very compatible with many other ingredients, and is thought to have good sliding properties and serve as a good solvent in oil-based formulas (Source) [3,4]. Because methyl perfluorobutyl ether contains perfluoroalkyl group (Rf) on one side and alkyl group (R) on the other side of ether linkage, it cannot be synthesized by selective partial fluorination of its hydrocarbon precursors. Instead, they may be prepared by coupling reactions of two reactants having Rf and R, coupling reactions of two reactants having Rf and R, respectively. For example:
- A batch reaction of heptafluorobutyryl fluoride (CF7COF) with dimethyl in the presence of potassium fluoride (KF) produce C4F9OCH3[5].
- C3F7COCl was added dropwise to a mixture of diglyme and KF at 50° over 30 min. Subsequently Me2SO4 was added dropwise to the reaction mixture at 70° over 1 h, and the reaction mixture was further stirred at 70° for 1 h to give 99% C4F9Ome [6].
- A batch reaction of the alkylating agent C3F7COF with methyl fluoride (CH3F) catalytic with anmony (Ⅴ) fluoride (SbF5) produce C4F9OCH3[7].
- The reversible addition of fluoride-ion to anhydrides of perfluoronated acids leads to formation of perfluoroalkoxy anions. The authors used C3F7COOH as the sources of perfluorinated O-anions, the corresponding chlorides were obtained from these acids by the action of PCl5. For alkylation of O-anions we used the set of alkylating agents. Firstly, the authors obtained Me perfluorononyl ether in 64% yield by the reaction of C3F7COCl with two moles of CsF in diglyme followed by addition of dimethylsulfate. And then, the authors obtained Me perfluorononyl ether in 70% yield by the reaction of C3F7COCl with two moles of CsF in diglyme followed by addition of CH3SO2OCH3[8].
References
[1] https://pubchem.ncbi.nlm.nih.gov/compound/Methyl-Nonafluorobutyl-Ether#section=3D-Conformer
[2] Hwang S, Kim J, Lee S, Lee D, Lee H, Sik H, Kim H. Preparation of methoxy and ethoxy nonfluorobutanes. J. Ind. Eng. Chem., 2007, 13(4): 537-544.
[3] http://www.ichemistry.cn/chemistry/163702-07-6.htm
[4] https://www.truthinaging.com/ingredients/methyl-perfluorobutyl-ether
[5] Flynn R, Grenfell M, Moore G, Owens J. Cleaning surfaces with perfluoroalkyl group-containing solvents. PCT Int. Appl. (1996), WO 9622356 A1 19960725.
[6] Takata, Naokado; Mochizuki, Takeshi; Fujimoto, Etsuo; Sekiya, Akira. Two-step preparation of fluoroethers from perfluoroacyl halides. Jpn. Kokai Tokkyo Koho (1998), JP 10045651 A 19980217.
);You may like
Related articles And Qustion
Lastest Price from METHYL NONAFLUOROBUTYL ETHER manufacturers
US $0.00-0.00/KG2024-03-25
- CAS:
- 163702-07-6
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- kg-tons

US $25.00-13.00/kg2023-12-11
- CAS:
- 163702-07-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons