What is Chlorotrimethylsilane?
Aug 10,2020
General Description
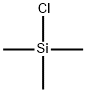
Chlorotrimethylsilane, also known as Trimethylsilyl chloride is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry. Trimethylsilyl chloride(TMCS) is a silyl chloride consisting of a central silicon atom covalently bound to one chloro and three methyl groups. Chlorotrimethylsilane is a derivatisation agent used in gas chromatography/mass spectrometry applications.
Chemical Properties
Trimethylchlorosilane is a colorless, fuming liquid. Irritating odor.Readily hydrolyzed with liberation of hydrogen chloride; soluble in benzene, ether and perchloroethylene.Boiling point 135°F Flash point -18°F. Density 0.854 g / cm3. The vapor and liquid may cause burns. Vapors are heavier than air.
Chlorotrimethylsilane reacts vigorously and exothermically with water to produce hydrogen chloride.

Uses
Chlorotrimethylsilane is a typical Silane Blocking Agent, which can protect or deprotect functional groups selectively. It is widely used in the syntheses of drugs. It have been used in the preparation of volatile derivatives of a wide range of compounds for GC analysis, and used for silylation and as a protection group in the process of various organic synthesis.
Trimethylsilyl chloride(TMCS) is the most extensively used derivatization reagent for gas chromatography.The reactions are generally performed under anhydrous conditions although reactions can be performed on samples obtained from water-containing samples.There are considerable opportunities for artifacts stemming from a variety of reasons including off-target reactions with aldehydes and ketones.The rate of reaction of TMCS with nucleophilic targets is comparatively slow compared to other alkylsilyl chlorides and requires the presence of a base catalyst such as pyridine. TMCS is included as a catalyst with other trialkylsilyl reagent such N,O-bis- (trimethylsilyl)trifluroacetamide (BSA) or N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA).The order of functional group reactivity is alcohols>phenols>carboxylic acids>amines>amides. Trimethylchlorosilane is used as an intermediate to make silicone products, including lubricants.
Preparation
TMSCl is prepared on a large scale by the Direct process, the reaction of methyl chloride with a silicon-copper alloy. The principal target of this process is dimethyldichlorosilane, but substantial amounts of the trimethyl and monomethyl products are also obtained.The relevant reactions are (Me = CH3):
x MeCl + Si → Me3SiCl, Me2SiCl2, MeSiCl3, other products Typically about 2-4% of the product stream is the monochloride, which forms an azeotrope with MeSiCl3.
Fire Hazard
Violent reaction with water. Toxic and irritating hydrogen chloride and phosgene may be formed in fires. Difficult to extinguish, re-ignition may occur. Flashback along vapor trail may occur.
Containers may explode in fire. Vapor may explode if ignited in enclosed area. When heated to decomposition or on contact with acids or acid fumes, chloride fumes are emitted. Reacts with surface moisture, releasing hydrogen chloride, which will corrode common metals and form flammable hydrogen gas. Avoid contact with water; Chlorotrimethylsilane readily hydrolyzes, liberating hydrochloric acid. Hazardous polymerization may not occur.
Toxicity
Similar to other silanes. Toxicity is rated high for inhalation, ingestion and local irritation. May cause death or permanent injury after a very short exposure to small quantities.
Poison by ingestion and skin contact. Moderately toxic by inhalation and intraperitoneal routes. A corrosive irritant to skin, eyes, and mucous membranes. Questionable carcinogen with experimental neoplastigenic data. Mutation data reported. A flammable liquid and very dangerous fire hazard when exposed to heat or flame. Violent reaction with water or hexafluoroisopropylideneamino lithium, A preparative hazard. To fight fire, use foam, alcohol foam, fog. When heated to decomposition it emits toxic fumes of Cl-. An intermediate in the production of silicones.
Safety Profile
Poison by ingestion and skin contact. Moderately toxic by inhalation and intraperitoneal routes. A corrosive irritant to skin, eyes, and mucous membranes. Questionable carcinogen with experimental neoplastigenic data. Mutation data reported. A flammable liquid and very dangerous fire hazard when exposed to heat or flame. Violent reaction with water or hexafluoroisopropylideneamino lithium, A preparative hazard. To fight fire, use foam, alcohol foam, fog. When heated to decomposition it emits toxic fumes of Cl-. An intermediate in the production of silicones.
);
You may like
Benzhydrol:Melting point,Uses,Hazards
Mar 22, 2024
Lastest Price from Chlorotrimethylsilane manufacturers
Trimethylchlorosilane

US $100.00/kg2024-02-27
- CAS:
- 75-77-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100 tons
Cholrotrimethyylsilane

US $6.00/kg2024-01-08
- CAS:
- 75-77-4
- Min. Order:
- 1kg
- Purity:
- 99.96%
- Supply Ability:
- 500ton

![446292-08-6 1H-isoindole-1,3(2H)-dione, 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]](httpss://img.chemicalbook.com/NewsImg/2020-2-17/warter_2020217161163682.jpg)