What is 4-Pyridinepropanol?
In 4-Pyridinepropanol molecule, the unpaired electrons of nitrogen are not involved in the resonance structure of the pyridine aromatic ring, i.e. perpendicular to the aromatic plain; this basic lone pair of electrons on nitrogen categorizes pyridine as a ligand that forms classical Werner complexes. For instance, the pyridine ring is capable of axial ligation of different macrocycle complexes such as metal phthalocyanine (M-Pc) and so on. The pyridine rings can undergo hydrogen bonding with polar species. The pyridine group also has a strong affinity toward metallic surfaces. Nevertheless, it still has drawbacks. It is highly hygroscopic and basic in its nature and very reactive and easily degrades upon UV irradiation. For example, the 4-pyridinepropanol solution would turn to dark reddish-brown at both 254 nm and 356 nm lights, while tolerant for light with energy less than 2.61 eV.1 In addition, the presence of –OH group allows the esterification of 4-pyridinepropanol with an acid. Therefore, 4-pyridinepropanol can serve as a reactant in reactions including the following ones:
➀The reaction between 4-pyridinepropanol and sodium formate can be used in preparation of ester-modified polybutadienes. But it’s worth noting that the heteroaromatic reactant reacts less efficiently than analogues such as chelation auxiliary 2-pyridinemethanol, et, al.2
➁The deoxyfluorination of 4-pyridinepropanol with crystalline N-tosyl-4-chlorobenzenesulfonimidoyl fluoride could reach a 4-(3-fluoropropyl)pyridine yield of 61%. 3
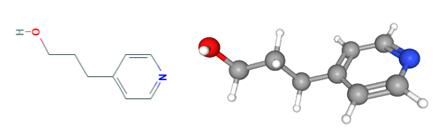
Fig 1. Chemical structure formula of 4-Pyridinepropanol
The application of 4-pyridinepropanol has been reported in the following studies1:
➀Because of 4-pyridinepropanol’s ability to covalently bond to the surface of silicon, 4-pyridinepropanol-Si (111) surface can be used as a cradle for immobilizing redox active.
➁Pyridine moiety of 4-pyridinepropanol molecule functionalized silicon surfaces is capable of attaching noble metal nanoparticles such as palladium, gold and silver with good adhesion.
References
1. Alavi, A.; Tahay, P.; Safari, N., Covalent immobilization of 4-pyridinepropanol monolayers on Si (111) surfaces: Axially ligation F16CoPc on pyridine moieties. Appl. Surf. Sci. 2019, 480, 235-242.
2. Park, W.-J.; Lee, C.-H.; Kim, D.-S.; Jun, C.-H., A catalytic hydroesterification process using HCO2Na, Ru3(CO)12 and alcohols for the preparation of ester modified polybutadienes. Chem. Commun. 2015, 51 (78), 14667-14670.
3. Guo, J.; Kuang, C.; Rong, J.; Li, L.; Ni, C.; Hu, J., Rapid Deoxyfluorination of Alcohols with N-Tosyl-4-chlorobenzenesulfonimidoyl Fluoride (SulfoxFluor) at Room Temperature. 2019, 25 (30), 7259-7264.
4. https://pubchem.ncbi.nlm.nih.gov/compound/4-Pyridinepropanol
5. http://www.chemspider.com/Chemical-Structure.65755.html?rid=1bfa7983-b529-4fa0-9d63-7e58b8de5d0d
See also
Lastest Price from 4-Pyridinepropanol manufacturers
US $1.10/g2021-10-27
- CAS:
- 2629-72-3
- Min. Order:
- 1g
- Purity:
- 99.00%
- Supply Ability:
- 100 Tons Min