ChemicalBook > Articles Catagory List >Pharmaceutical-intermediates >What-is-4-Fluorobenzyl-bromide-
What-is-4-Fluorobenzyl-bromide-
Feb 14,2020
4-Cyanobenzyl bromide (C8H6BrN, CAS registry No. 17201-43-3) is a white solid. Its melting point is 113-117 oC, and flash point is 125.1 °C. It is insoluble in water at 20 °C. It is stable under normal temperatures and pressures. It should be stored at 0-5°C.
4-Cyanobenzyl bromide is an important intermediate for pharmaceutical production. It can be used for the synthesis of a series of piperidine-linked aromatic diimidazolines, which have been synthesized as conformationally restricted congeners of the anti-Pneumocystis carinii (PCP) drug, Pentamidine. These compounds are synthesized from 4-cyanobenzyl bromide, which was then heated with triethyl phosphite to 120 °C for 8 h to give 4-cyanobenzyl phosphonate as synthetic precursors[1]. These resulting compounds significantly inhibited the growth of Pneumocystis carinii in culture at 1 μg/mL. 4-Cyanobenzyl bromide can also be used for the synthesis of 1-(4-Cyanobenzyl)-4-[[(trans-iodopropen-2-yl)oxy]methyl]piperidine, which has been synthesized as a novel iodinated ligand for sigma receptors. This new compound is synthesized from 4-hydroxymethypiperidine and 4-cyanobenzyl bromide in the presence of K2CO3 at 40 oC[2]. This new compound exhibited high affinity for the sigma-l receptor and selectivity for sigma-l vs. sigma-2 receptors using in vitro receptor binding assays.
In addition, 4-cyanobenzyl bromide can be used for the synthesis of 1,3-bis(p-benzyl substituted)-4,5-bisaryl-imidazolium halides with 4,5-bisaryl-1H-imidazole[3A, 3B]. Theses symmetrically substituted N-heterocyclic carbene (NHC) precursors can react with silver(I) acetate to give symmetrically p-benzyl-substituted 4,5-diaryl-imidazole N-heterocyclic carbene-silver(I) acetate complexes, which have shown some promising cytotoxic results against the human cancer cell lines Caki-1 and MCF-7; the cytotoxicity is already comparable to platinum-based drugs on these specific cell lines. In addition, significant antibacterial activity against E. coli and S. aureus were observed but the level of conventional antibiotics was not reached yet for the derivatives described. Moreover, 4-cyanobenzyl bromide can be used as alkylating agent to give corresponding intermediates for the synthesis of a series of rationally designed benzylindolyldiketo acids acting as potent HIV-1 integrase strand transfer inhibitors[4]. 4-Cyanobenzyl bromide can also be used as the initiator for the atom transfer radical polymerization using activators generated by electron transfer (AGET ATRP) of styrene in a FeCl3·6H2O/PPh3 /VC catalytic system[5].
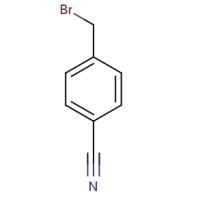
Fig 1. Chemical structure formula of 4-cyanobenzyl bromide
References
[1].Huang, T. L.; Qian, Z.; White, A. T.; Queener, S. F.; Bartlett, M. S.; Smith, J. W.; Donkor, I. O., Synthesis and anti-pneumocystis carinii activity of piperidine-linked aromatic diimidazolines. Bioorganic & Medicinal Chemistry Letters 1996, 6 (17), 2087-2090.
[2].Waterhouse, R. N.; Mardon, K.; O'Brien, J. C., Synthesis and preliminary evaluation of [123I]1-(4-cyanobenzyl)-4-[[(trans-iodopropen-2-yl)oxy]-methyl]piperidine: A novel high affinity sigma receptor radioligand for SPECT. Nuclear Medicine and Biology 1997, 24 (1), 45-51.
[3].[A]Hackenberg, F.; Lally, G.; Müller-Bunz, H.; Paradisi, F.; Quaglia, D.; Streciwilk, W.; Tacke, M., Novel symmetrically p-benzyl-substituted 4,5-diaryl-imidazole N-heterocyclic carbene-silver(I) acetate complexes – Synthesis and biological evaluation. Journal of Organometallic Chemistry 2012, 717, 123-134; [B]Hackenberg, F.; Lally, G.; Müller-Bunz, H.; Paradisi, F.; Quaglia, D.; Streciwilk, W.; Tacke, M., Synthesis and biological evaluation of N-heterocyclic carbene–silver(I) acetate complexes derived from 4,5-ditolyl-imidazole. Inorganica Chimica Acta 2013, 395, 135-144.
[4].Ferro, S.; Barreca, M. L.; De Luca, L.; Rao, A.; Monforte, A. M.; Debyser, Z.; Witvrouw, M.; Chimirri, A., New 4-[(1-Benzyl-1H-indol-3-yl)carbonyl]-3-hydroxyfuran-2(5H)-ones, β-Diketo Acid Analogs as HIV-1 Integrase Inhibitors. Archiv der Pharmazie 2007, 340 (6), 292-298.
[5].Liang, Z.; Fangli, Y.; Fang, L., AGET ATRP of Styrene Using 4- Cyanobenzyl Bromide as the Initiator. Journal of Yancheng Institute of Technology(Natural Science Edition) 2015, 28 (2), 9-14.
);
You may like
Is PQQ better than vitamin C or CoQ10?
Jan 24, 2024
Lastest Price from 4-Cyanobenzyl bromide manufacturers
4-Cyanobenzyl bromide

US $0.00/KG2023-11-15
- CAS:
- 17201-43-3
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
4-Cyanobenzyl bromide

US $10.00/KG2022-06-02
- CAS:
- 17201-43-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 tons