What is 2,3,4-Trifluoro-5-nitrobenzoic acid?
2,3,4-Trifluoro-5-nitrobenzoic acid (2,3,4-Trifluoro-5-nitro-benzoic acid; C7H2F3NO4) as heterocyclic compound is an important intermediate compound in process of preparing pharmaceutical agents such as benzimidazole derivatives [1].
2,3,4-Trifluoro-5-nitrobenzoic acid was commonly synthesized by nitration reaction of 2,3,4-trifluorobenzoic acid, which are well known to those skilled in the art (Fig.1). In general, fuming nitric acid was added dropwise into ice-cooled 2,3,4-trifluorobenzoic acid in present of concentrated sulfuric acid as activating agent at 5 to 30 °C, and the mixture was stirred at the same temperature for 5 hours to complete the reaction [2]. Then, the product was obtained by multi-steps treating process, including ice-cooled, ethyl acetate, water washed, dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness. 2,3,4-Trifluorobenzoic acid in the mixture sulphuric acid and hexamethyldisiloxane was added a 1:1 mixture of sulphuric acid (96 wt %) and nitric acid (98 wt %) slowly at 15 and 25° C. The mixture was stirred for a further 5 hours and then run onto ice to provide 2,3,4-trifluoro-5-nitrobenzoic acid with a higg yield of 74.3% [1]. Moreover, 2,3,4-tTrifluorobenzoic acid was added to the mixture of sulfuric acid and potassium nitrate under ice cooling, then stirred overnight at room temperature, which also could produce 2,3,4-trifluoro-5-nitrobenzoic acid successfully [3].
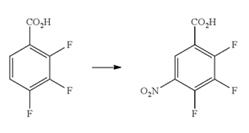
Fig.1 The synthesis of 2,3,4-trifluoro-5-nitrobenzoic acid.
2,3,4-Trifluoro-5-nitrobenzoic acid as intermediate could further react to produce other important heterocyclic compounds. For example, a hydrogen gas was passed through a mixed solution of 2,3,4-trifluoro-5-nitrobenzoic acid, ethanol and Pd/C (50% hydrated material) under nitrogen atmosphere at room temperature to carry out the reaction. After reaction, 5-amino-2,3,4-trifluorobenzoic acid would be obtained [4]. 4-Amino-2,3-difluoro-5-nitrobenzoic acid could be synthesized by the substitution reaction of 2,3,4-trifluoro-5-nitrobenzoic acid and concentrated ammonium hydroxide (28%), which could be further used to prepare benzimidazole compounds [5]. After methyl esterification of 2,3,4-trifluoro-5-nitrobenzoic acid, it can occur substitution reaction at 2,4-sites with ammonium hydroxide solution to obtain methyl 2,4-diamino-3-fluoro-5-nitrobenzoate [6]. Moreover, 2,3,4-trifluoro-5-nitrobenzoic acid also could occur substitution reaction at 2,4-sites with ammonium hydroxide solution directly in N-methylpyrrolidinone at 80-90 °C, and produce 2,4-diamino-3-fluoro-5-nitrobenzoic acid [1]. 3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-nitro-benzoic can be prepared by the 2- substitution reaction of 2,3,4-trifluoro-5-nitrobenzoic acid with 2,3,4-trifluoro-5-nitrobenzoic acid under certain conditions,which also an synthetic intermediate for preparing N-methyl-substituted benzamidazoles [7]. In conclusion, the above-mentioned trifluoro-substituted benzoic acid derived from 2,3,4-trifluoro-5-nitrobenzoic acid are useful as a starting material for preparing of benzimidazoles, such as quinolone carboxylic acids useful as, for example, a medicine, particularly as an anti-bacterial or antiviral agent.
References
[1] DeMattei, J., Shakya, S., Piscopio, A. D., Hache, B. P., Evans, M. C., Ford, J. G., ... & Leonard, J. (2013). U.S. Patent No. 8,501,956. Washington, DC: U.S. Patent and Trademark Office.
[2] Yamamoto, Y., Yoneda, Y., Ataka, K., & Yokota, N. (2001). U.S. Patent No. 6,307,091. Washington, DC: U.S. Patent and Trademark Office.
[3] Yazaki, A., Yoshida, J., & Niino, Y. (1999). U.S. Patent No. 5,994,575. Washington, DC: U.S. Patent and Trademark Office.
[4] Yamamoto, Y., Yoneda, Y., Ataka, K., & Yokota, N. (2000). U.S. Patent No. 6,160,171. Washington, DC: U.S. Patent and Trademark Office.
[5] DeMattei, J., Shakya, S., Nichols, P. J., Barnett, B. R., Hache, B. P., Evans, M. C., ... & Leonard, J. (2012). U.S. Patent No. 8,183,385. Washington, DC: U.S. Patent and Trademark Office.
[6] https://pubchem.ncbi.nlm.nih.gov/compound/15871241
[7] http://www.chemspider.com/Chemical-Structure.11216209.html?rid=57b33fbb-2dca-4297-a338-ee4642588980
You may like
See also
Lastest Price from 2,3,4-Trifluoro-5-Nitro-Benzoic Acid manufacturers

US $0.00-0.00/kg2023-12-24
- CAS:
- 197520-71-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1 ton
US $0.00-0.00/kg2022-09-23
- CAS:
- 197520-71-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton

![904316-92-3 3-Oxa-8-azabicyclo [3.2.1] octane, hydrochloride (1:1); Synthesis; Synthetic intermediate](httpss://www.chemicalbook.com/NewsImg/2020-1-9/20201910572510598.jpg)