What is 1,3,5-Trifluorobenzene?
Using a single-mode argon laser as the excitation light source, the rotating Raman spectrum of 1,3,5-trifluorobenzene was studied at high resolution. The proton and fluorine magnetic resonance spectra of 1,3,5-trifluorobenzene in nematic liquid crystal were analyzed.
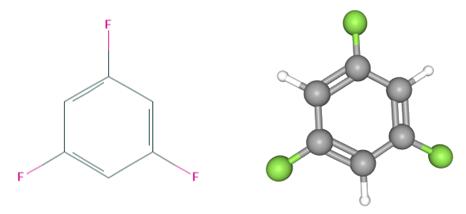
Fig 1. Chemical structure formula and three-dimensional structure of 1,3,5-Trifluorobenzene
During gas-phase catalytic hydrogenation of trichlorotrifluorobenzenes, defluorination proceeds along with dechlorination to form considerable amounts of difluorobenzenes, besides the target trifluorobenzenes. Degrees of defluorination are from 50% to 95%. However, when carrying out the reactions of dechlorination under milder conditions in the liquid phase using a carbon-based 2% Pd catalyst in a solution of butanol, a mixture of trifluorobenzenes is formed. The mixture of the isomers of trifluorobenzenes formed by the dechlorination reaction is separated by rectification to recover 1,3,5-trifluorobenzene[1].
It is known that in the commercial production of hexafluorobenzene by interaction of hexachlorobenzene with potassium fluoride, incompletely fluorinated polyfluoro-polychlorobenzenes are formed as by-products. The fraction, containing isomeric trifluorotrichlorobenzenes, consists of 65–75% symmetric 1,3,5-trichloro-2,4,6-trifluorobenzene, and the other two isomers are present from 25 to 35%。Thus, taking account of the comparatively readily available raw materials base, the selective substitution of chlorine atoms for hydrogen in polyfluorochlorobenzenes without affecting the fluorine atoms is of interest as a route polyfluorobenzenes which are not readily available. One of the possible methods of such a conversion is a reductive dehalogenation of polyfluorobenzenes. Catalytic dehalogenation of aromatic halogen derivatives is known [2]. We have carried out an investigation of the process of dechlorination of the isomers of trifluorotrichlorobenzenes by hydrogen using carbon-based 2% Pd catalyst under vapor-phase and liquid-phase conditions.The reactor was charged with 118 g (0.5 mol) of a mixture of the isomers of trichlorotrifluorobenzenes (the content of 1,3,5-trichloro-2,4,6-trifluorobenzene was 60%), 600 ml of butanol-1, 25 g of a finely divided catalyst (2% Pd/C) and 150 g of sodium acetate (CH3COONa.3H2O). The stirrer was started and, after preliminary purging of the system with nitrogen, the hydrogen feed through the bubbler was started. The hydrogen feed rate was adjusted so that a small breakthrough of hydrogen through the reaction mass was provided. The reaction mixture was heated to a temperature of 80–90°C. The reaction products and the solvent entrained by the hydrogen flow to the trap were condensed. The process was complete when the starting trichlorotrifluorobenzene was absent in the reactor and the content of mono-and dihydrotrifluorobenzenes did not exceed 15%, usually after 40–60 h. Then the crude product was filtered from catalyst, combined with the contents, of trap and rectified. The rectification was carried out in a Fischer rectification column collecting the fraction boiling at a temperature of 75–80°C with ∼80% content of 1,3,5-trifluorobenzene, as shown by GLC. Repeated rectification gave 31 g of 1,3,5-trifluorobenzene[2-4].
1,3,5-trifluorobenzene is also used in the synthesis of (-)-epicatechin and its 3-O-gallate.
References
[1] Finger G C , Reed F H , Finnerty J L . Aromatic fluorine compounds. V. 1,3,5-Trifluorobenzene[J]. Journal of the American Chemical Society, 1951, 73(1):152-153.
[2] Shipilov A I , Kostareva E V , Kochanov A S . Selective dechlorination of polychlorofluoroaromatic compounds: preparation of 1,3,5-trifluorobenzene[J]. Journal of Fluorine Chemistry, 1999, 96(1):23-24.
[3] Gentle I R , Ritchie G L D . Second hyperpolarizabilities and static and optical-frequency polarizability anisotropies of benzene, 1,3,5-trifluorobenzene and hexafluorobenzene[J]. The Journal of Physical Chemistry, 1989, 93(22):7740-7744.
[4] https://pubchem.ncbi.nlm.nih.gov/compound/9745
[5] http://www.chemspider.com/Chemical-Structure.21111798.html?rid=be9e8e88-6cd6-4955-b72a-eb46210fa35d
You may like
See also
Lastest Price from 1,3,5-Trifluorobenzene manufacturers
US $100.00-1.00/KG2024-03-25
- CAS:
- 372-38-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available
US $0.00-0.00/kg2022-10-11
- CAS:
- 372-38-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton