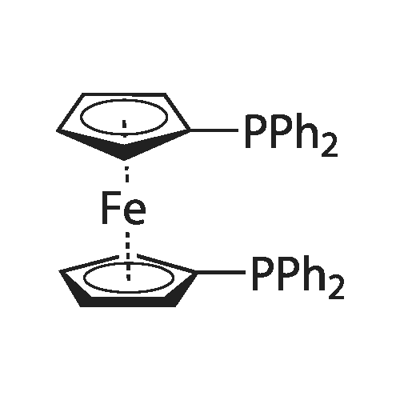
What is 1,1'-Bis(diphenylphosphino)ferrocene?
General description
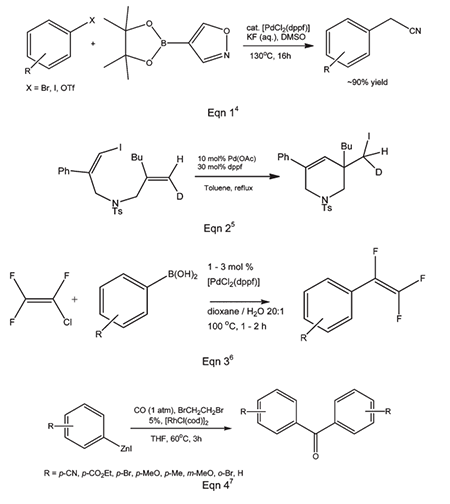
Novel functionalized furan derivatives were prepared via Pd-phosphine sequential C-C and C-O bond formation.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency.
Application
Ligand for ruthenium-catalyzed greener amine synthesis from amines and alcohols by hydrogen-borrowing.
Ruthenium-Catalyzed N-Alkylation of Amines and Sulfonamides Using Borrowing Hydrogen Methodology
Ligand used in a Pd(II)-catalyzed cross-coupling synthesis of oxazepine ring systems.
Instances
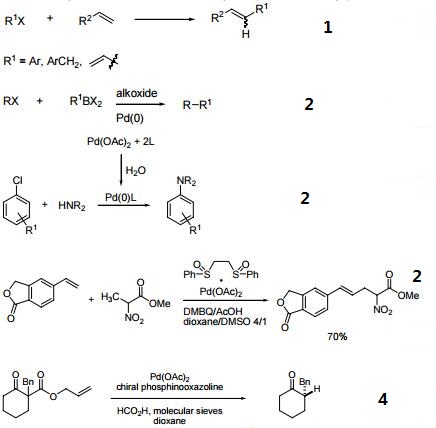
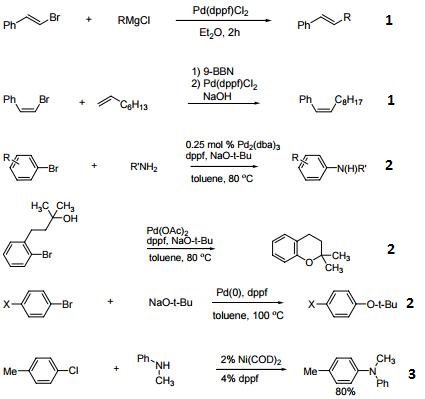
1. Ligand for Pd-catalyzed cross-coupling.
2. Useful ligand for Pd-catalyzed carbon-nitrogen and carbon-oxygen bond forming procedures.
3. Ligand for Ni-catalyzed amination of aryl chlorides.
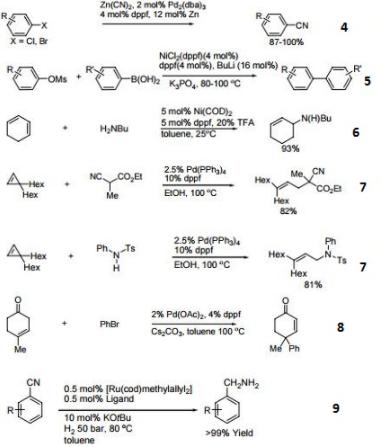
4. Ligand for Pd-catalyzed conversion of aryl halides to aryl nitriles.
5. Ligand for Ni-catalyzed Suzuki reactions.
6. Ni-catalyzed hydroamination of 1,3-dienes.
7. Pd-catalyzed hydrocarbonation and hydroamination of 3,3-dihexylcyclopropene.
8. Pd-catalyzed γ-arylation of β,γ-unsaturated ketones.
9. Ligand for Ru-catalyzed reduction of nitriles to primary amines.
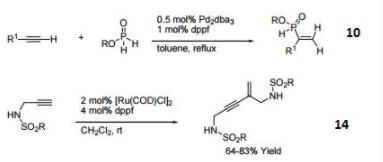
10. Ligand for Rh-catalyzed alkyne head-to-tail dimerization.
11. Ligand for Rh-catalyzed cross-coupling
12. Ligand for Rh-catalyzed olefin isomerization
13. Ligand for Ni or Rh-catalyzed borylation
14. Ligand for regioselective Pd-catalyzed hydrophosphinylation of terminal alkynes to form branched alkenes.
Synthesis of Carbinols
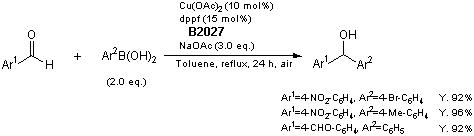
Typical procedure: Under an air atmosphere, a Schlenk tube is charged with Cu(OAc)2·H2O (4.0 mg, 0.02 mmol), dppf (16.6 mg, 0.03 mmol), arylboronic acid (0.4 mmol), aldehyde (0.2 mmol), NaOAc (49.2 mg, 0.6 mmol), and toluene (3 mL) under ice-salt (-20 °C). The mixture is stirred for 0.5 h at room temperature, refluxed for 24 h, and then cooled in a Schlenk tube to room temperature. The mixture is extracted with ethyl acetate (4×5 mL), and the organic layers are washed with water. The organic layers are dried over MgSO4, concentrated, and purified by flash column chromatography on silica gel to give the desired product.
);You may like
Related articles And Qustion
Lastest Price from 1,1'-Bis(diphenylphosphino)ferrocene manufacturers

US $5.00-2.00/kg2024-04-16
- CAS:
- 12150-46-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available
US $35.00-286.00/g2024-02-23
- CAS:
- 12150-46-8
- Min. Order:
- 100g
- Purity:
- 0.98
- Supply Ability:
- 100kg