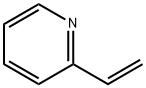
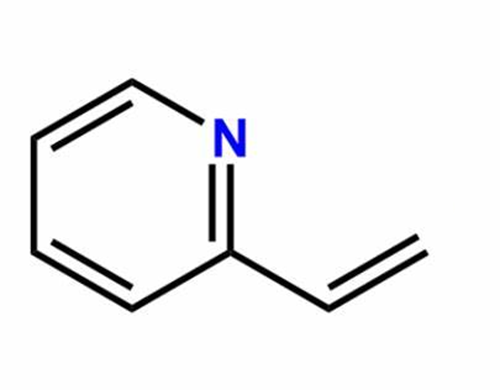
Application of 2-Vinylpyridine
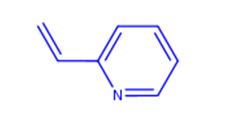
2-Vinylpyridine (Betahistine Impurity 1(Betahistine EP Impurity A); 2-Vinylpyridine, Stab. With 0.1% 4-Tert-Butylcatechol; 2-Vinylpyridine, stabilized, 97% 5ML; 2-ethenyl-pyridin; 2-Pyridyle thyle ne; 2-vinyl-pyridin; 2-Ethenylpyridine; 2-VINYLPYRIDINE)[1] is an organic compound, widely used in the chemical yield. It’s quite unstable and may be stabilized with a small amount of ter-butyl catechol, and heat sensitive - store cold. Flammable. Incompatible with strong oxidizing agents, strong bases, strong acids. In general, this compound is an dark brown liquid with an unpleasant odour[1].
2-Vinylpyridine is a pyridine deriviative used in the preparation of poly (2-vinylpyridine) via radical polymerization. 2-Vinylpyridine is an impurity of Betahistine (B324000). Some metalloorganic or metal amide compounds of very electropositive metals, such as lithium, sodium, beryllium, magnesium, and aluminum, cause the polymerization of 2‐vinylpyridine[2]. Whereas the compounds of beryllium and of aluminum furnish slightly crystalline 2‐vinylpyridine polymers, by using magnesium derivatives it is possible to obtain polymers which are highly crystalline by x‐rays. 4‐Vinylpyridine, in the presence of catalysts which are stereospecific for the polymerization of 2‐vinylpyridine, gives amorphous noncrystallizable polymers. the nitrogen atom, in the case of 4‐vinylpyridine is too far from the vinyl double bond to form, with the catalyst, an addition complex able to give the same orientation to all the monomer molecules, at the very moment of the polymerization[2].
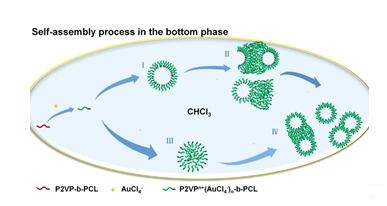
At the same time, 2-Vinylpyridine is also used to improve effectiveness of protective polymer coatings on metals. Sakir et al. found that Ag nanostructures were prepared by direct surface-growth from Pt nanoparticles that were immobilized on Si substrates modified with a layer of end-grafted poly (2-vinylpyridine)[3]. In addition, 2-Vinylpyridine is also popularly used in self-assembly. Self-assembly behavior of amphiphilic block copolymers has attracted much attention recently. Li et al. discovered that An aqueous solution of HAuCl4 and a chloroform solution of poly (2-vinylpyridine)-block -polycaprolactone (P2VP199-b-PCL22) are employed as the upper phase and the bottom phase, respectively. Structural transitions from toroids and plate like structures to vesicles and large compound vesicles are observed in the bottom phase by using transmission electron microscopy (TEM)[4].
References
[1] https://www.chemicalbook.com/ProductChemicalPropertiesCB6852593_EN.htm
[2] Natta G, Mazzanti G, Longi P, et al. Stereospecific polymerization of 2‐vinylpyridine[J]. Journal of Polymer Science, 1961, 51(156): 487-504.
[3] Sakir M, Onses M S. Solid substrates decorated with Ag nanostructures for the catalytic degradation of methyl orange[J]. Results in Physics, 2019, 12: 1133-1141.
[4] Li X, Li S, Zou H, et al. Block copolymer vesicles via liquid/liquid interface-mediated self-assembly[J]. Applied Surface Science, 2020, 499: 143896
You may like
Related articles And Qustion
See also
Lastest Price from 2-Vinylpyridine manufacturers

US $1.00/kg2024-02-27
- CAS:
- 100-69-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 300MT/month

US $0.00-0.00/kg2023-12-27
- CAS:
- 100-69-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1 ton