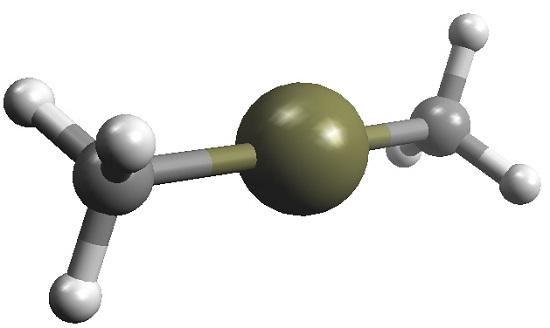
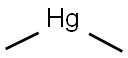Use and Environmental Fate of dimethylmercury
The first indication of the extreme toxicity of dimethylmercury (DMM) was documented in 1863 when two laboratory assistants died of DMM poisoning while synthesizing DMM in the laboratory of Frankland and Duppa. There are numerous reports of people dying from alkyl mercury compounds including a chemist who was preparing several thousand grams ofDMMin his laboratory in 1974. The extreme toxicity was revisited in 1997, when Karen Wetterhahn, an internationally renowned researcher of the carcinogenic effects of heavy metals on DNA repair proteins, died within a few months after a single exposure of less than a milliliter of DMM on her latex-covered hand. DMM is extremely toxic and lethal at a dose of approximately 400 mg of mercury (equivalent to a few drops) or about 5mgkg-1 of body weight or as little as 0.1 ml.

Use
Dimethylmercury has limited use because of its toxicity but can be used to calibrate research equipment, as in its application as a standard reference material for 199Hg NMR measurements.
Mechanism of Toxicity
In contrast to the white crystalline solids of the pure forms of
methylmercury (MMM) and phenylmercury, Dimethylmercury exists as
a colorless liquid at room temperature with high volatility.
These physical qualities enable high concentrations of the
substance to be absorbed by exposure pathways of the skin and
lungs that circumvent first-pass elimination. Effectively, this
prolongs the systemic circulation of DMM, and extends its
residence time in the body.
The additional alkyl group flanking the mercury imparts
DMM with lipophilicity that exceeds its monoalkylated
counterpart, and allows DMM to be sequestered in lipid-rich
depots. The metabolic delay allows the neurotoxicity of DMM
to remain latent for months.
The gradual conversion into MMM results in the release of
DMM from depots such as lipid-rich tissues and plasma
proteins, and permits its movement through barriers such as
the blood–brain and placenta. A cysteine complex of the
monomethylated metabolite penetrates the endothelial cells of
the blood–brain barrier by mimicking methionine and using
the large neutral amino acid transporter.
Thus, the toxicity of DMM is mediated by its dealkylation.
Cleavage of the carbon–mercury bond generates MMM
metabolites, which can form covalent bonds with cellular
ligands with amphiphilic properties. The mercury center reacts
with sulfur and sulfur-containing thiol groups of enzymes
and thereby inhibits them, resulting in cellular dysfunction.
The metal center of DMM acts as a soft acid, and binds tightly
to polarizable donor atoms in soft bases. An additional
mechanism of adverse effect is the disruption of the prooxidant–
antioxidant balance, causing oxidative damage to
biomolecules resulting cellular damage. Within cells, mercury
may interact with a variety of proteins, particularly microsomal
and mitochondrial enzymes. Recent studies demonstrated that
the combined administration of the antioxidants N-acetyl
cysteine, zinc, and selenium mitigated DMM acute and chronic
toxicity by reducing enzymatic and cellular dysfunction.
Environmental Fate
Dimethylmercury is a colorless liquid that is volatile at room temperature (vapor pressure 62.3 mmHg) and is slightly soluble in water (water solubility 8860 mg l-1). There are no reports on the partition behavior of DMM but it is known to readily evaporate and is thus rarely found in sediment or soil. No reports were found on the environmental persistence of DMM. While DMM vaporizes, no studies were found on long range transport. The lipophilicity ofDMMresults in its accumulation inadipose tissue, plasma proteins, and brain. DMM has not been found in fish.
);You may like
Related articles And Qustion
Lastest Price from DIMETHYLMERCURY manufacturers

US $300.00/kg2023-09-07
- CAS:
- 593-74-8
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 50000 tons