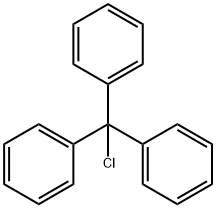
The uses of Triphenylchloromethane
Triphenylchloromethane is a reagent used for the protection of amines, carboxylic acids,alcohols, and thiols.The Handling, Storage, and Precaution: use chemical safety goggles and rubber gloves. Do not breathe dust. Avoid prolonged or repeated exposure. Wash thoroughly after handling. It is an irritant. Keep tightly closed in a cool, dry place. Incompatible with strong oxidizing agents as well as strong bases.
Toxic decomposition product is hydrogen chloride gas.
Preparation
Triphenylmethyl chloride is commercially available. It may be prepared by the reaction of triphenylmethanol with acetyl chloride, or by the Friedel-Crafts alkylation of benzene with carbon tetrachloride to give the trityl chloride-aluminium chloride adduct, which is then hydrolyzed.
Uses
Protection of Alcohols. Triphenylchloromethane (trityl chloride, TrCl) is most commonly used as a reagent to protect primary alcohols as the corresponding trityl ethers. Because of the stability of trityl ethers under neutral or basic conditions, their facile removal under mildly acidic conditions and significant steric and lipophilic nature, they have been used quite extensively over the past 70 years. The classical method to prepare trityl ethers using TrCl involves reaction of the alcohol in pyridine at temperatures ranging from room temperature to 100 ◦C. Recent examples using this procedure include the preparation of a 6-O-trityl glucopyranoside (eq 1)and a protected purine nucleoside (eq 2). The first example was detritylated later in that synthesis with pToluenesulfonic Acid (p-TsOH) in CH2Cl2/MeOH while the second was deprotected with Trifluoroacetic Acid (TFA) in CH2Cl2.
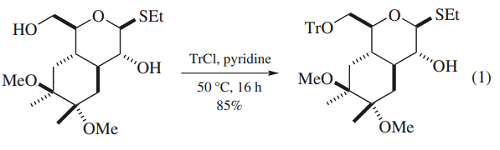
Both examples illustrate the observation that primary alcohols are routinely regioselectively protected in the presence of secondary alcohols with TrCl, presumably due to the steric requirements of this reagent. While trityl ethers have been used extensively in nucleoside chemistry, it has been noted that the trityl group is not appropriate for the protection of purine deoxyribonucleosides as their removal leads to depurination; 4-Methoxytrityl Chloride and 4,4 -Dimethoxytrityl Chloride are the reagents of choice for this application.
References
1. Greene, T. W.; Wuts, P. G. M., Protective Groups in Organic Synthesis; Wiley: New York, 1999, p 102.
2. De Kort, M.; Regenbogen, A. D.; Overkleeft, H. S.; Challiss, R. A. J.; Iwata, Y.; Miyamoto, S.; van der Marel, G. A.; van Boom, J. H., Tetrahedron 2000, 56, 5915.
3. Ichikawa, S.; Shuto, S.; Matsuda, A., J. Am. Chem. Soc. 1999, 121, 10270.
4. Schaller, H.; Weimann, G.; Lerch, B.; Khorana, H. G., J. Am. Chem. Soc. 1963, 85, 3821.
);You may like
Related articles And Qustion
See also
Lastest Price from Triphenylmethyl Chloride manufacturers

US $0.00/Kg/Bag2024-04-17
- CAS:
- 76-83-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200mt
US $8.00-0.80/KG2024-03-25
- CAS:
- 76-83-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available