The biological properties of Gallic acid
Background
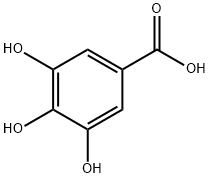
Gallic acid (GA) is a component of hydrolyzable tannins, also known as gallic acid, chemical name 3,4,5-trihydroxybenzoic acid. The content of gallic acid in Yunnan Pu-erh tea (picture 1) is relatively high, and it has various biological effects such as antibacterial, anti-inflammatory, anti-tumor, and anti-mutation. Gallic acid itself has a significant antioxidant effect, and also has many functions such as anti-tumor, killing trypanosomes, protecting the liver and anti-hepatitis B virus; recent studies have pointed out that gallic acid may be an effective ingredient for Pu-erh tea to inhibit the synthesis of cholesterol in HepG2 cell lines one.

Picture 1 Yunnan Pu-erh tea
Anti-inflammatory activity of gallic acid
Gallic acid was found to possess antiinflammatory activity towards zymosan-induced acute food pad swelling in mice. In vitro studies on the mode of action of gallic acid revealed that this compound interferes with the functioning of polymorphonuclear leukocytes (PMNs). Scavenging of superoxide anions, inhibition of myeloperoxidase release and activity as well as a possible interference with the assembly of active NADPH-oxidase may account for the inhibition of inflammatory process by gallic acid. Structure-activity relationship analysis showed that the o-dihydroxy group of gallic acid is important for the inhibitory activity in vitro.
Azadirachta indica bark is the main ingredient in the preparation of the ayurvedic drug Nimba arishta which is applied in Sri Lanka to skin diseases (rashes) and as blood-purifier [1]. By activity-guided fractionation of an aqueous extract of Azadirachta bark one of the compounds we ended up with was gallic acid which was found to inhibit luminol-dependent chemilumines-. cence generated by zymosan-stimulated human polymorphonuclear leukocytes (PMNs). Consequently, the inhibition of chemiluminescence by Nimba arishta was found to correlate with the gallic acid content of the preparation. Inhibition in the chemiluminescence assay may be indicative of anti-inflammatory activity in vivo.
This paper deals with the anti-inflammatory activity of gallic acid and its possible mode of action. Gallic acid was tested in vivo in a zymosan-induced foot pad inflammation model. In vitro studies were focussed on PMN functions such as the generation and transformation of O and other reactive oxygen species (ROS). Structural requirements for the expression of anti-inflammatory activity by gallic acid analogues will be presented.
Materials and Methods
Five times-concentrated veronal saline buffer, pH 7.35 (VSB-5x), prepared according to Mayer, served as a stock solution for the preparation of VSB', containing 0.5mM Mg2 and 0.15mM Ca2, and for EGTA-VB, containing 2.5mM Mg2, and 8mM ethylenebis(oxyethylenenitrilo)- tetraacetic acid (EGTA, Aldrich). Chemiluminescence assay: Experiments were performed in Hank's balanced salt solution buffered at pH 7.35 with NaHCO, (HBSS, Gibco, Paisly, Scotland). Before use, 0.1 % (w/v) of gelatin was added to the buffer to avoid cell aggregation (HBSS-gel). Zymosan-induced inflammation Effects of gallic acid on zymosan-induced inflammation were studied in groups of five male BALB/c mice of 10—12 weeks. Inflammation was induced by injecting 300 pg of zymosan (Sigma Chemicals Co., St. Louis MO, USA), suspended in 25 p1 of sterilized saline in the left hind foot pad. Gallic acid (Carl Roth OHG, Karlsruhe FRG) dissolved in 500 p1 of saline was administered intra-peritoneally (i.p.) immediately before and 3 hours after zymosan injection. Control mice were injected with saline. Foot pad swelling was measured 6 hours after induction. The thickness of the foot pad of individual mice before zymosan injection served as control. Erythrocytes Sheep and rabbit blood in citrate-buffered glucose (Alsever's solution) served as sources of sheep erythrocytes (ShE) and rabbit erythrocytes (RaE), respectively. To elute possibly adsorbed serum proteins, the erythrocytes were washed three times with 0.16M sodium iodide before use. ShE were resuspended in VSB2 (4 x 108 cells/mi), and sensitized by incahation with an equal volume of 1: 2000 diluted rabbit anti-ShE serum (hemolytic amboceptor) at room temperature for 10mm (the sensitized cells are referred to as ShEA). Subsequently the mixture was centrifuged and ShEA were resuspended in VSB' (4 x io cells/mI). RaE were resuspended in EGTA-VB (3 x 108 cells/mi)
Hemolytic assay for human complement activity
Human pooled serum (HPS) from five healthy volunteers was used as source of complement. Classical pathway (CP) and alternative pathway (AP) activities were determined by a modified version of the microassay described by Klerx et al. The test was performed in U-well microtitre plates (no. 651101; Greiner Labor-technik, Nurtingen, FRG). Logarithmic dilutions of gallic acid were prepared in VSB2 (CP) or EGTA-VB (AP). To each well containing 100 p1 of these dilutions, 25 p1 of 10_16 dilution of HPS in VSB2 (CP) or 25 p1 of a 1002 dilution of HPS in EGTA-VB (AP) were added after which the microtitre plates were preincubated for 30mm at 37°C. Subsequently, 25 p1 of ShEA (4 x 108 cells/mi VSB2*) (CP) or RaE (uncoated) (3 x 108 cells/mi EGTA-VB) (AP) were added. The plates were incubated at 37°C for 60 mm (CP) or 30 mm (AP). After incubation, the plates were centrifuged at 3000 rpm for 5mm to precipitate intact cells and cell ghosts. To determine the degree of hemolysis, 5Opl of the supernatants were mixed with 200 p1 of water in 96 wells flat-bottom microtiter plates (no 655101; Gremner). The light absorbance at 405 nm was measured using an automatic Elisa reader (STL instruments, model SF plus). Controls in this assay consisted of similarly treated supernatants of erythrocytes incubated with water (100% hemolysis), or VSB2, EGTA-VB, or heat-inactivated (56°C for 30 mm) serum dilutions (0% hemolysis), or buffer supplemented with the appropriate dilution of HPS (0% inhibition).
Reference
You may like
See also
Lastest Price from Gallic acid manufacturers

US $0.00/kg2024-04-11
- CAS:
- 149-91-7
- Min. Order:
- 1kg
- Purity:
- 99.8%
- Supply Ability:
- 1000 kg

US $0.00/KG2024-04-09
- CAS:
- 149-91-7
- Min. Order:
- 1KG
- Purity:
- ≥98% HPLC
- Supply Ability:
- 1000KG