Synthesis and one Application of p-Toluenesulfonic acid
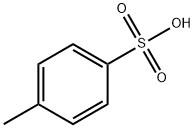
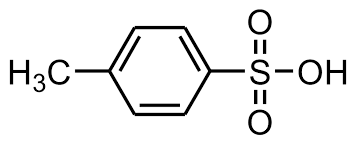
p-Toluenesulfonic acid (PTSA or pTsOH) or tosylic acid (TsOH) is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The CH3C6H4SO2– group is known as the tosyl group and is often abbreviated as Ts or Tos. Most often, TsOH refers to the monohydrate, TsOH.H2O.
As with other sulfonic acids, TsOH is a strong organic acid. It is about one million times stronger than benzoic acid. It is one of the few strong acids that is solid and, hence, conveniently weighed.
Synthesis
p-Toluenesulfonic acid monohydrate is prepared by sulfonation of toluene with concentrated sulfuric acid in a Dean Stark setup for water removal. The reaction mixture is worked up by formation of the monohydrate and recrystallization. The crude product is dissolved in a minimum of water that is then saturated with gaseous HCl to reduce the solubility of the product, which crystallizes out on cooling. The product is dried over NaOH and sulfuric acid to remove both water and HCl. Yield is 57.6% based on sulfuric acid of a slightly off-white powder melting at 99-103°C (lit m.p. 103-106°C).
Application
A new procedure for analyses for tryptophan in proteins and glycoproteins is described. The method utilizes p-toluenesulfonic acid as the catalyst for hydrolysis and the amino acid analyzer for the quantitative estimation of tryptophan. The hydrolysis of proteins is carried out in 3 N p-toluenesulfonic acid containing 0.2% 3-(2-aminoethyl)-indole in evacuated sealed tubes at 110° for 22, 48, and 72 hours. The values of tryptophan obtained were close to the expected integral values and the recoveries of all other amino acids were comparable to those observed after hydrolysis with 6 N HCl.
Since the hydrolysate can be placed on the ion exchange column, without the prior removal of the solvent which is required when 6 N HCl is used for the hydrolysis, the present procedure is more convenient than the use of 6 N HCl. This procedure, however, is not designed for the analyses of proteins that are grossly contaminated with carbohydrates such as feeds. The limit of carbohydrate content that the present procedure can tolerate is 2.0 mg when 2 to 4 mg of protein are being hydrolyzed with 1.0 ml of the acid.
);You may like
See also
Lastest Price from p-Toluenesulfonic acid manufacturers

US $10.00/kg2024-04-24
- CAS:
- 104-15-4
- Min. Order:
- 1kg
- Purity:
- 99.8%
- Supply Ability:
- 10000ton

US $6.00/KG2024-04-12
- CAS:
- 104-15-4
- Min. Order:
- 1KG
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/Month