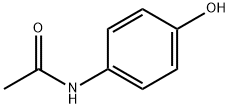
Paracetamol: not as safe as we thought?
Paracetamol is the most widely used over-the-counter and prescription analgesic worldwide.1 It is the first step on the WHO pain ladder and is currently recommended as first-line pharmacological therapy by a variety of international guidelines for a multitude of acute and chronic painful conditions.2
The mechanism of paracetamol's analgesic action remains largely unknown, but recent studies demonstrate that paracetamol inhibits prostaglandin production within the central nervous system and within peripheral tissues.3 Irrespective of its efficacy, it is generally considered to be safer than other commonly used analgesics such as non-steroidal anti-inflammatory drugs (NSAIDs) or opiates.
The analgesic benefit of paracetamol has recently been called into question in the management of one chronic painful condition, osteoarthritis (OA).4 Clinicians and patients need up-to-date evidence of benefits and harms to make evidence-based decisions on pharmacological prescription, and a recent estimate of the true risks of paracetamol at standard analgesic doses has not been available. We therefore conducted a systematic review of studies investigating the association between paracetamol and major adverse events (AEs) in the general adult population to provide a clinically informative toxicity profile.
Methods
Data sources and study selection
We followed recommendations made by the Meta-analysis of Observational Studies in Epidemiology and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) groups.5 ,6
We searched Medline and Embase for English-language studies published from database inception to 7 May 2013. The full search strategy was limited to only identify observational studies and can be found in the online supplementary material. All relevant references were checked for additional citations. Randomised controlled trial (RCT)-level evidence was not considered a meaningful way of capturing AE data because of the short-term follow-up of RCT trial participants as well as strict eligibility for trial entry, meaning that the general population would not be represented. If cohort-level evidence was found for an AE outcome, case–control evidence was not considered.
Studies were eligible for inclusion if they met the predefined protocol: the study population was adults aged >18 years and the study investigated one or more of the AEs of interest when people were taking oral paracetamol at a standard therapeutic dose of 0.5–1 g every 4–6 h to a maximum of 4 g/day compared with non-use.
Outcomes
The main outcomes investigated were all-cause mortality, cardiovascular AEs (specifically incidence of myocardial infarction, cerebrovascular accidents and hypertension), gastrointestinal (GI) AEs (specifically incidence of GI bleeding) and renal AEs (specifically reductions in estimated glomerular filtration rate (eGFR), increases in serum creatinine concentration and the need for renal replacement therapy).
We first screened titles and abstracts, and one reviewer (SB) screened relevant full-text articles. The second reviewer (VDN) reviewed 10% of the full-text articles screened, which were selected at random. One reviewer (SB) extracted study characteristics and adjusted summary statistics with 95% CIs and recorded the data in a standard form. Two authors (SB and ER) independently assessed the study quality using Grading of Recommendations Assessment, Development and Evaluation (GRADE). Each outcome is given a quality rating of high, moderate, low or very low based upon risk of bias, inconsistency, indirectness and imprecision. Risk of bias for each outcome was assessed using checklists for observational studies, which are based on the Strengthening the Reporting of Observational Studies in Epidemiology statement.7 GRADE clinical evidence profiles for each outcome can be found in the online supplementary material.
Statistical analysis
Studies that met the inclusion criteria and reported summary statistics with 95% CIs, or presented sufficient data for the calculation of summary statistics and 95% CIs, were considered for inclusion in meta-analysis. Where data were able to be pooled, heterogeneity was assessed using the χ2 and I2 statistics. Heterogeneity was predefined as χ2 p<0.1 or I2 >50%, and where heterogeneity was unable to be removed by predefined subgroups a random effects model was assumed and outcomes were downgraded in quality. In instances where data were unable to be pooled, due to difference in outcome or paracetamol dosage reporting, individual adjusted summary statistics were presented for each outcome per study. We produced forest plots to visually assess the summary statistics and 95% CIs of each study; analyses were done with Review Manager Version Five.
Results
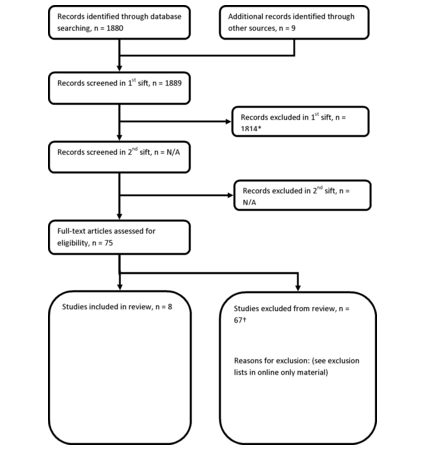
The search process identified 1888 records. Eight studies met the inclusion criteria, all of which were cohort studies. As all prespecified outcomes were found from these eight cohort studies, no case–control evidence was considered. Figure 1 shows the PRISMA flow chart for study selection. Table 1 reports the included study characteristics and results. The quality of evidence varied between outcomes and was graded as low or very low across all outcomes. Due to the non-comparable nature of outcomes and different paracetamol dosage definitions reported by individual studies, meta-analysis was only possible for a singular outcome; the incidence of hypertension. For all other outcomes, individual adjusted summary statistics are presented by AE category.
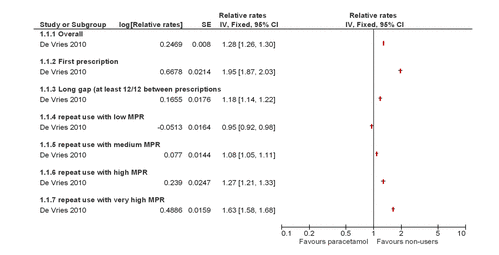
Two studies reported mortality,8 ,9 of which one reported a dose–response increase in the relative rate of all-cause mortality,8 and one reported a significantly increased standardised mortality ratio for those patients prescribed paracetamol compared with those not prescribed paracetamol as shown in figure 2.9
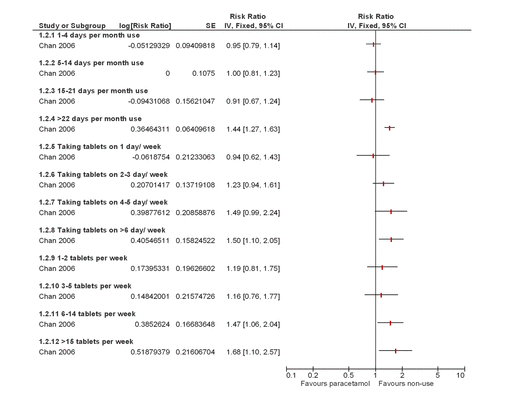
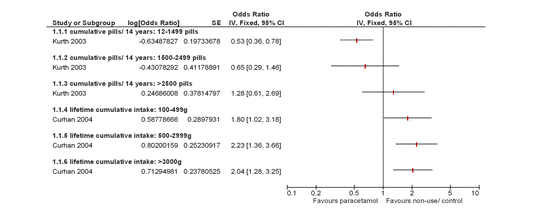
Four studies reported cardiovascular AEs,8 ,10–12 all of which demonstrated a dose–response. One study reported a dose–response increase in the risk ratio of cardiovascular AEs (confirmed or probable non-fatal myocardial infarction, non-fatal stroke, fatal coronary heart disease or fatal stroke) as shown in figure 3;10 one study reported a dose–response increase in the relative rate of the new cases of myocardial infarction and stroke;8 and two studies reported a dose–response increase, which remained when data were pooled, in the relative risk of new cases of hypertension for those patients taking paracetamol compared with those not taking paracetamol.11 ,12
One study reported GI AEs,8 which showed a dose–response increase in the relative rate of upper GI AEs (gastroduodenal ulcers and complications such as upper GI haemorrhages) for those patients prescribed paracetamol compared with those not prescribed paracetamol as shown in figure 4.
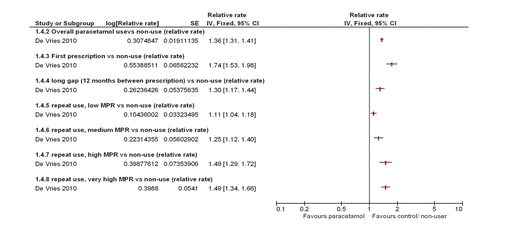
Four studies reported renal AEs,8 ,13–15 three of which demonstrated a dose–response. One study reported a dose–response increase in OR of a decrease of ≥30 mL/min/1.73 m2 in eGFR and ≥30% decrease in eGFR as shown in figure 5;13 one study reported a dose–response increase in the number of new cases of acute renal failure;8 one study reported a dose–response increase in OR of ≥0.3 mg/dL increase in serum creatinine and a decrease of ≥30 mL/min/1.73 m2 in eGFR;15 and one study reported no dose–response relationship in the estimated progression rates of chronic kidney disease and no difference in time to renal replacement therapy between those taking paracetamol and those not taking paracetamol.14
Discussion
The objective of this review was to synthesise the long-term observational evidence of the harmful effects of paracetamol. Findings from this systematic review demonstrate a consistent dose–response relationship between paracetamol at standard analgesic doses and AEs that are often observed with NSAIDs. This includes a dose–response relationship between paracetamol and increasing incidence of mortality, cardiovascular, GI and renal AEs in the general adult population.
The main limitations of this study are the low number of studies and quality of available evidence. As all studies included were observational, the GRADE system of quality rating per outcome begins at ‘low’ quality and can subsequently be upgraded or downgraded per individual outcome. This initial ‘low’ rating is based purely on the study's observational designs and does not take into consideration that observational studies are the most appropriate study design to assess the risk of long-term AE outcomes. Five of the studies were conducted in healthy female registered nurses or male physicians, which may limit the generalisability of the data to the general population.10–13 ,15
Although the sample size of most included studies was large, and the reported dosage regimens were consistent with modern dosage regimens, reliance on self-reported medication use and channelling bias with incomplete adjustment for potential confounders may have had an important impact. Four studies did not adjust for concomitant NSAID use, and channelling bias may lead those patients deemed unsuitable for NSAID therapy to be prescribed paracetamol as a ‘safer’ alternative,8 ,9 ,12 ,14 thus creating an allocation bias to a preselected group of patients with higher risk of AEs. Often referred to in the literature as ‘confounding by indication’, the indication and choice of analgesic treatment by clinicians may be related to the risk of future health outcomes and result in an imbalance of the underlying risk profile between paracetamol and non-paracetamol groups, potentially leading to biased results. All confounders adjusted for in each study can be found in the online supplementary material table S1. Six studies described self-reported medication use10–15 and two studies8 ,9 used only paracetamol prescription data. Both of these methods have the potential to inaccurately estimate the true amount of taken paracetamol in the studied cohort. A further limitation of the review is the various definitions of paracetamol dosage regimen across studies. Some report lifetime intake, while others report the number of pills or grams of paracetamol per unit time. This prevented the quantitative pooling of different doses in meta-analysis and the ability to draw firm conclusions as to safe dosage regimens.
While these limitations are important to consider, the striking trend of dose–response is consistent across multiple outcomes and studies. There is also evidence from the case–control literature supporting the dose–response seen in the current review,16 and a similar toxicity profile is demonstrated in systematic reviews of short-term RCTs.4
Several large observational studies confirm a better side effect profile for paracetamol compared with traditional NSAIDs.17 ,18 In line with the findings of the current review, one such study has also shown that the combination use of paracetamol and NSAIDs significantly increased the number of hospitalisations for upper GI AEs.18 In keeping with our findings, the addition of gastroprotective agents to paracetamol prescription significantly reduced this event compared with paracetamol alone. As well, a recent RCT comparing paracetamol and ibuprofen in a population of patients with knee pain showed 14/192 (7%) patients in the paracetamol arm experienced a haemoglobin drop of ≥1 g/dL at day 10, and by week 13 this figure rose to 20%,19 which was not significantly different from the drops in haemoglobin observed in the ibuprofen-only group.
Every prescribing decision involves a calculation of risk versus benefit, a trade-off of efficacy versus tolerability. If providing adequate analgesia or antipyresis, clinicians and patients may be willing to accept the risk at the level of AEs demonstrated in this review. However, when analgesic benefit is uncertain, as has been recently suggested for paracetamol in the treatment of OA joint pain4 and low back pain, more careful consideration of its usage is required.20 Prescribers need to be aware of patients’ individual responses to paracetamol and the observed increased toxicity with regular and higher dosing within standard analgesic dose ranges. Based upon the data presented above, we believe the true risk of paracetamol prescription to be higher than that currently perceived in the clinical community. Given its high usage and availability as an over-the-counter analgesic, a systematic review of paracetamol's efficacy and tolerability in individual conditions is warranted.
);You may like
Related articles And Qustion
See also
Lastest Price from Acetaminophen manufacturers

US $15.00/kg2024-04-16
- CAS:
- 103-90-2
- Min. Order:
- 1kg
- Purity:
- 99.912%
- Supply Ability:
- 10ton

US $0.00-0.00/kg2024-04-16
- CAS:
- 103-90-2
- Min. Order:
- 25kg
- Purity:
- 98%
- Supply Ability:
- 1000kg