Ozone-Hazard and Toxicity
Description
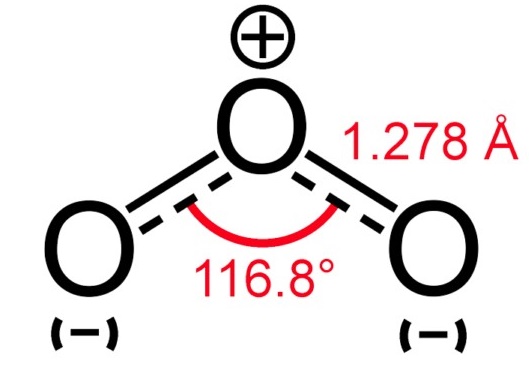
Ozone,O3, a colorless gas or dark blue liquid, also known as activated oxygen,is an allotropic form of oxygen formed in nature by lightning in air and during evaporation of water particularly by spray in the sea.It is an unstable blue gas with a distinctive odor. Ozone absorbs ultraviolet rays and acts as a natural blanket that protects the earth from harmful short-wave radiation from the sun. Ozone is a powerful oxidizer. It is used as an oxidant in the rubber industry, as a bleaching agent,as a water purifier, and to treat industrial wastes.
Toxicity Data
LC50 inhal (rat) 4.8 ppm (4 h)
PEL (OSHA) 0.1 ppm (0.2 mg/m3)
TLV-TWA (ACGIH) 0.1 ppm (0.2 mg/m3)
STEL (ACGIH) 0.3 ppm (0.6 mg/m3)
Major Hazards
Extremely irritating to the eyes and respiratory tract; high acute toxicity. Reacts violently with many oxidizable organic and inorganic substances; may form shocksensitive and highly explosive reaction products. High concentrations of ozone are a fire and explosion hazard when in contact with anyorganic substance that can be oxidized.
In moderately high concentrations ozone is very toxic when inhaled, and in lesser concentrations,it is irritating to the nose and eyes. Ozone in the lower atmosphere contributes to airpollution and smog. It can cause damage to rubber, plastics, and paints. These low concentrationscan cause headaches, burning eyes, and respiratory irritation. It is particular harmful toasthmatics and the elderly with respiratory problems.
Toxicity
Ozone is a highly toxic gas that is extremely irritating to the eyes, mucous membranes, and respiratory tract. The characteristic odor of ozone can be detected below the permissible exposure limit, and this compound is therefore regarded to have adequate warning properties. However, at higher concentrations the ability to smell ozone may decrease. Inhalation of 1 ppm ozone may cause headaches and irritation of the upper and lower respiratory tract.
The first symptoms of exposure include irritation of the eyes, dryness of throat, and coughing; these symptoms disappear after exposure ceases. Exposure at higher levels may lead to lacrimation, vomiting, upset stomach, labored breathing, lowering of pulse rate and blood pressure, lung congestion, tightness in the chest, and pulmonary edema, which can be fatal. Exposure to 100 ppm of ozone for 1 hour can be lethal to humans. Animal studies indicate that chronic exposure to ozone may result in pulmonary damage, leading to chronic lung impairment. Continual daily exposure to ozone can cause premature aging.
Flammability and Explosibility
Ozone by itself is not flammable. Liquid ozone and concentrated solutions are extremely hazardous and can explode on warming or when shocked.
Reactivity and Incompatibility
Ozone is a powerful oxidant and can react explosively with readily oxidizable substances and reducing agents. Explosions can occur when ozone is exposed to bromine, hydrogen bromide, hydrogen iodide, nitrogen oxides, lithium aluminum hydride, metal hydrides, hydrazine, alkyl metals, stilbene, ammonia, arsine, and phosphine.
Ozone reacts with alkenes and other unsaturated organic compounds to form ozonides, many of which are highly unstable and explosive. Ozone combines with many aromatic compounds and ethers to form shock-sensitive and explosive products.
Storage and Handling
In particular, work with ozone should be conducted in a fume hood to prevent exposure by inhalation. Ozone is usually produced in the laboratory with a ozone generator, and care should be taken to ensure adequate ventilation in the area where the ozone generation equipment is located. Because of the possibility of the generation of explosive ozonides, ozonolysis reactions should always be conducted in a fume hood behind a safety shield.
Accidents
An ozone leak can be easily detected by its characteristic pungent odor. If a large amount of ozone is inhaled, move the person to fresh air and seek medical attention at once. In the event of eye contact, promptly wash eyes with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention.
Respiratory protection may be necessary in the event of an accidental release of ozone.
Disposal
Ozone is usually produced on demand from a laboratory ozone generator, and a procedure for the treatment of excess ozone should be included in the experimental plan. Small to moderate amounts of excess ozone can be vented to the fume hood or other exhaust system. When large amounts of excess ozone are anticipated, the excess gas should be passed through a series of traps containing a 1 to 2% solution of potassium iodide or other reducing agent before venting to the fume hood.
);