Methanol-Hazard and Toxicity
Description
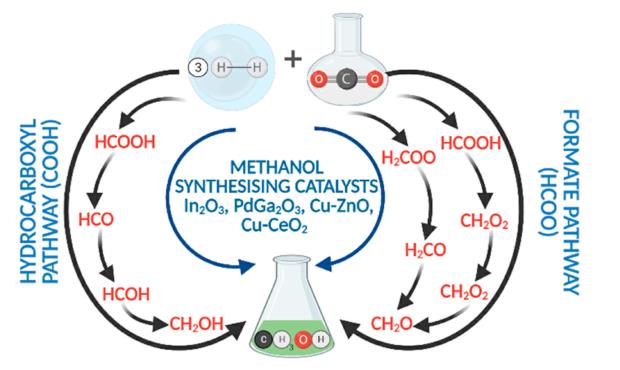
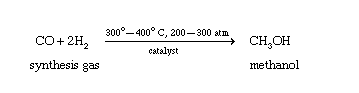
Methyl alcohol, also known as methanol or wood alcohol, is a clear, colorless, flammable liquid that is the simplest alcohol. World production of methanol is approximately 8.5 billion gallons annually. Methanol is produced industrially, starting with the production of synthesis gas or syngas. Syngas used in the production of methyl alcohol is a mixture of carbon monoxide and hydrogen formed when natural gas reacts with steam or oxygen. Methyl alcohol is then synthesized from carbon monoxide and hydrogen.
Methyl alcohol is poisonous and is commonly used to denature ethyl alcohol. Methanol poisoning results from ingestion, inhalation of methanol vapors, or absorption through the skin. Methanol is transformed in the body to formaldehyde (H2CO) by the enzyme alcohol dehydrogenase.The formaldehyde is then metabolized to formic acid (HCOOH)by aldehyde dehydrogenase.
Toxicity Data
LD50 oral (rat) 5628 mg/kg
LD50 skin (rabbit) 15,840 mg/kg
LC50 inhal (rat) >145,000 ppm (1 h)
PEL (OSHA) 200 ppm (260 mg/m3)
TLV-TWA (ACGIH) 200 ppm (260 mg/m3)—skin
STEL (ACGIH) 250 ppm (328 mg/m3)
Major Hazards
Highly flammable liquid; low acute toxicity. Exposure to excessive vapor causes eye irritation, head- ache, fatigue and drowsiness. High concentrations can produce central nervous system depression and optic nerve damage. 50,000 ppm will probably cause death in 1 to 2 hrs. Can be absorbed through skin. Swallowing may cause death or eye damage.
Toxicity
The acute toxicity of methanol by ingestion, inhalation, and skin contact is low. Ingestion of methanol or inhalation of high concentrations can produce headache, drowsiness, blurred vision, nausea, vomiting, blindness, and death. In humans, 60 to 250 mL is reported to be a lethal dose. Prolonged or repeated skin contact can cause irritation and inflammation; methanol can be absorbed through the skin in toxic amounts. Contact of methanol with the eyes can cause irritation and burns. Methanol is not considered to have adequate warning properties.
Methanol has not been found to be carcinogenic in humans. Information available is insufficient to characterize the reproductive hazard presented by methanol. In animal tests, the compound produced developmental effects only at levels that were maternally toxic; hence, it is not considered to be a highly significant hazard to the fetus. Tests in bacterial or mammalian cell cultures demonstrate no mutagenic activity.
Flammability and Explosibility
Methanol is a flammable liquid (NFPA rating = 3) that burns with an invisible flame in daylight; its vapor can travel a considerable distance to an ignition source and "flash back." Methanol-water mixtures will burn unless very dilute. Carbon dioxide or dry chemical extinguishers should be used for methanol fires.
Reactivity and Incompatibility
Methanol can react violently with strong oxidizing agents such as chromium trioxide, with strong mineral acids such as perchloric, sulfuric, and nitric acids, and with highly reactive metals such as potassium. Sodium and magnesium metal react vigorously with methanol.
Storage and Handling
In particular, methanol should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If methanol is ingested, obtain medical attention immediately. If large amounts of this compound are inhaled, move the person to fresh air and seek medical attention at once.
In the event of a spill, remove all ignition sources, soak up the methanol with a spill pillow or absorbent material, place in an appropriate container, and dispose of properly. Respiratory protection may be necessary in the event of a large spill or release in a confined area.
Disposal
Excess methanol and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
);Related articles And Qustion
Lastest Price from Methanol manufacturers
US $50.00-1.00/KG2024-03-25
- CAS:
- 67-56-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $2.00/kg2024-02-13
- CAS:
- 67-56-1
- Min. Order:
- 10000kg
- Purity:
- 99%
- Supply Ability:
- 10000000