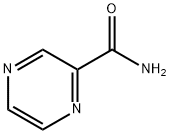
Mechanism of action of Pyrazinamide
Pyrazinamide (pyrazine-2-carboxamide) is a first-line antituberculous agent critical to the success of short-course chemotherapy of tuberculosis. It is a derivative of nicotinamide, first synethesized in 1952 and subsequently shown to possess a high degree of antituberculosis activity in man. The activity of pyrazinamide is restricted to the Mycobacterium tuberculosis complex – all isolates of M. bovis are resistant. Pyrazinamide is available as a pure tablet in strengths of 150, 400, or 500mg under multiple trade names and also as a part of fixed drug combinations with rifampicin and isoniazid.
Its mechanism of action of is still disputed, but pyrazinamide has a sterilizing action and treatment-shortening effect in vivo, probably related to bactericidal activity on semidormant M. tuberculosis within acidic microenvironments.

Mechanism of action
Pyrazinamide is a prodrug that is converted into the active form, pyrazinoic acid, within M. tuberculosis by the action of pyrazinamidase, a nicotinamidase encoded by the gene pncA. Pyazinoic acid accumulates intracellularly at acid pH, suggesting a possible mechanism for the particular activity of pyrazinamide within acidic environments. Mutations in the pncA gene of M. tuberculosis result in resistance to pyrazinamide, and many different mutations in pncA have been described, including in the upstream intergenic region. Mycobacterium bovis is naturally resistant to pyrazinamide because all strains have a mutation in pncA. The absence of pyrazinamidase activity can be used to detect pyrazinamide resistance in M. tuberculosis. Strains of M. tuberculosis with a wild-type pncA sequence have been described that have phenotypically low-level pyrazinamide resistance but retain pyrazinamidase activity and efflux mechanisms are also thought to play a role in mediating such resistance.
The specific cellular target of pyrazinamide remains controversial. Pyrazinoic acid inhibits the mycobacterial fatty acid synthase I (FAS-I), which is an essential enzyme for mycobacterial survival responsible for the synthesis of long-chain fatty acids from acetyl-CoA precursors that are then used as precursors for mycolic acid synthesis. Mycolic acids are an important component of the lipid-rich mycobacterial cell wall and their synthesis is inhibited by isoniazid and ethionamide. Others have disputed these findings and it may be that the mechanism of action is due to nonspecific cellular acidification impairing multiple vital processes.
Excretion About 4% of administered pyrazinamide is excreted as such in the urine and 30–41% as pyrazinoic acid. Urinary concentrations of pyrazinamide, similar to serum levels, reach a peak in about 2 hours then fall exponentially from 3 to 48 hours after a dose. The half-life of the drug in the urine is about 9 hours. The ratio of urinary to serum concentrations of pyrazinamide after a single dose is fairly constant; after a dose of 1.5 g urinary concentrations are about 44% higher and after 3.0 g about 85% higher. Approximately 98% of pyrazinamide filtered by the kidneys is reabsorbed but very little pyrazinoic acid appears to be reabsorbed. Urinary levels of pyrazinoic acid after a single dose of pyrazinamide reach a maximum after about 12 hours and fall exponentially from 16 to 50 hours.
Toxicity
This is the most important toxic effect of this drug. Hepatotoxicity first became evident in the USA when pyrazinamide was used as a first-line drug for tuberculosis in the 1950s. In these early studies a high dosage of 40–50 mg/kg body weight per day was used. Results of a United States Public Health Service Trial (1959) indicated that hepatotoxicity was related to the use of such high dosage. As a result of these and similar studies, the use of pyrazinamide as a first-line drug for tuberculosis was abandoned. Later it became apparent that the reputation of pyrazinamide as a toxic drug had been exaggerated because of the circumstances in which it had been used.
Apart from being used in high dosage for prolonged periods, it was often used with other toxic drugs such as ethionamide and/or cycloserine in retreatment regimens, frequently in elderly patients, who are more susceptible to hepatotoxicity. When pyrazinamide was used in later studies in a moderate daily dosage of 20–30 mg/kg body weight, hepatic toxicity was not considered a major problem. Moreover, even when pyrazinamide was used in high dosage in intermittent regimens (maximum of 90 mg/kg weekly) combined with streptomycin or streptomycin plus isoniazid, hepatotoxicity was uncommon.
);You may like
Lastest Price from Pyrazinamide manufacturers

US $1.00/KG2023-11-15
- CAS:
- 98-96-4
- Min. Order:
- 1KG
- Purity:
- 99%~101.0% ,BP2015
- Supply Ability:
- 5ton/month
US $1.10/g2021-07-17
- CAS:
- 98-96-4
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min