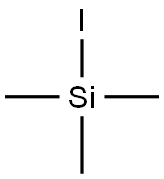Iodotrimethylsilane : A Mild and Efficient Reagent for the Reduction of Azides to Amines
The reduction of azides to amines is an important reaction for the synthesis of a variety of organic compounds. The azides can be prepared with good regio, stereo, and enantioselectivity and their transformation to the amino group provides a wide scope of applications in organic synthesis. As a result, a number of reagents have been reported in the literature I for this reductive process. An exhaustive review 2 has described various aspects of such reductions. Some of the recent reagents include the borohydrides 3-5 benzyltriethylammonium tetrathiomolybdate 6, hexamethyldisilathiane 7,8 and samarium iodide 9,1°. Inspite of a large number of methods reported for this conversion there has been considerable interest to explore more milder and selective reagents.
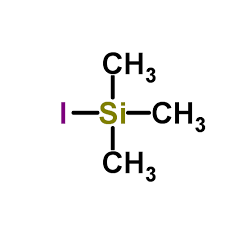
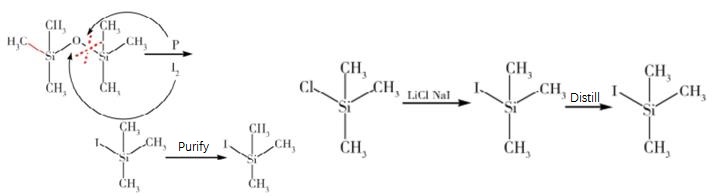
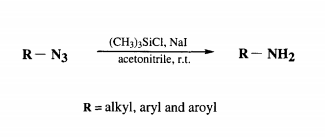
In the last two decades an explosive growth has been witnessed in the use of organo-silicon reagents in organic synthesis. Iodotrimethylsilane with remarkable reactivity as a hard-soft reagent has been known for a wide spectrum of synthetic applications ll,12. Literature studies 13,14 have demonstrated that in situ generation of iodotrimethylsilane using chlorotrimethylsilane-sodium iodide reagent system is desirable because of its near neutral conditions, more reactive nature and commercial viability from practical point of view is, in comparisian to the use of iodotrimethylsilane directly. In our recent studies on the synthesis of some natural products, we were confronted with the challenge of exploring a mild and versatile method for the reduction of azido group wi~Lhout affecting the other susceptible functionalities. In this connection, we report an efficient reduction of alkyl, aryl and aroyl azides upon treatment with chlorotrimethylsilane and sodium iodide (in situ generation of iodotrimethylsilane) in acetonitrile at room temperature to provide the corresponding primary amines and amides. To our knowledge there is no previous precedent reported for this type of reduction with iodotrimethylsilane or by it's in situ generation. The results have been summarised in the Table. Similar results have also been obtained by using iodotrimethylsilane directly.
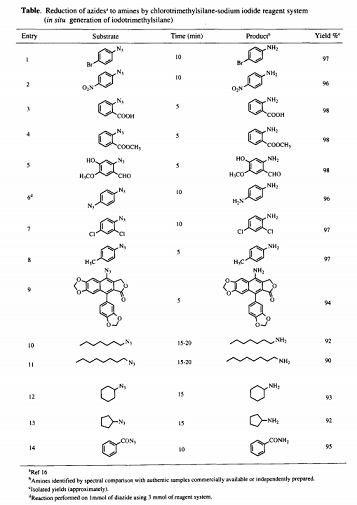
In a typical procedure; to a solution of 2-azido benzoic acid ( 1 mmol) in acetonitrile (10 ml), sodium iodide (1.5 mmol) was added and stirred for 5 minutes. To this stirred suspension, a solution of chlorotrimethylsflane (1.5 mmol) in acetonitrile (2 ml) was added dropwise and the stirring continued for another 10 minutes. O11 the completion of the reaction as indicated by TLC (ethylacetate-hexane, 7:3), the reaction mixture was quenched with 10% sodium thiosulphate solution and it was extracted with ethyl acetate (20 ml). The organic layer was washed with brine solution, dried over anhydrous Na2SO4 and concentrated under vacuum to give the crude product. This was purified by column chromotography (ethyl acetate-hexane, 1:1) to afford pure 2-amino benzoic acid (anthranilic acid) as white solid ( 98% yield).
Various azides 16 upon reduction in presence of iodotrimethylsilane gives the corresponding amines in almost quantitative yields (above 90%). These reactions are spontaneous and are found to be almost complete within few minutes as monitored by TLC. Moreover, this reagent is known in the literature for the cleavage of esters 17 as well as ethers 18. Intrestingly, under the present conditions this reagent did not cleave the ether or ester functionality, hence providing a selective azido reduction (entries 4 & 5). Further, as a part of the ongoing medicinal chemistry programme this selective reduction has also been applied for the reduction ef 4- azidopodophyllum lignan (entry 9) to it's corresponding amine without the opening of y-lactone ring, though this reagent is known for 7-1actone ring cleavage 19. In contrast to other azido reducing agents 2,9, this reagent does not affect the aldehyde, nitro and halo functionalities (entries 1, 2, 5 & 7). The secondary alkyl azides (entries 12 & 13) have also given the corresponing amines in good yields. Furthermore, unlike the only other organo-silicon reagent known for aromatic azide reduction i.e, HMDST 7, this reagent has broader applicability as it reduces the alkyl, aryl and as well as aroyl azides in excellent yields as seen in the Table.
The present approach which is carried out under extremely mild and neutral conditions has provided a novel as well as efficient and chemoselective protocol for the reduction of alkyl/aryl azides to the corresponding amines. The reagent employed for this process is rather inexpensive, non-stinking and non-toxic. Our present method compares favourably to most of the earlier literature reported procedures. Though this reagent is extensively employed in organic synthesis, this new finding further broadens its versatility.
References and Notes
1.Ito, M.; Koyakumaru, K.; Takaya, T. H. Synthesis 1995, 376.
2.Scriven, E. F. V.; Turnbull, K. Chem. Rev. 1988, 88, 297.
3.Rolla, F. J. Org. Chem. 1982, 47, 4327.
4.Rao, H. S. P.; Siva, P. Synth. Commun. 1994, 24, 549.
5.Alvarez, S. G.; Fisher, G. B,; Singavam, B. Tetrahedron Lett., 1995, 36, 2567.
);You may like
Related articles And Qustion
Lastest Price from Iodotrimethylsilane manufacturers

US $0.00-0.00/kg2023-08-11
- CAS:
- 16029-98-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 50000kg

US $100.00-50.00/KG2023-08-08
- CAS:
- 16029-98-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg