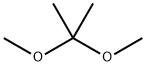
Introduction of 2,2-Dimethoxypropane
Formation of Acetals.
The reagent can be used to prepare a variety of acetals via transacetalization. This involves interchanging the alkoxy or ketone groups in an acidic medium. Both symmetrical [R1R2C(OR3)2] and mixed [R1R2C(OR3)OR4] acetals can be obtained, depending upon the choice of conditions.1
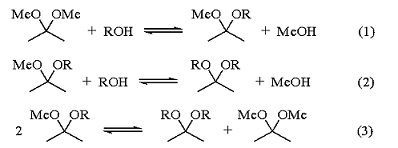
The three equilibria shown in eqs 1-3 are established when 2,2-dimethoxypropane, an alcohol, and a catalytic amount of acid are mixed. Distillation of the methanol formed shifts the equilibrium far in the direction of the new acetals. Methanol and 2,2-dimethoxypropane form a binary azeotrope; however, this can be broken by the addition of a hydrocarbon solvent such as hexane or benzene.
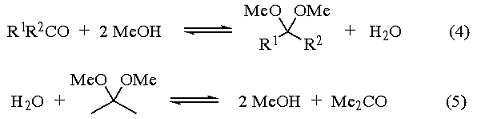
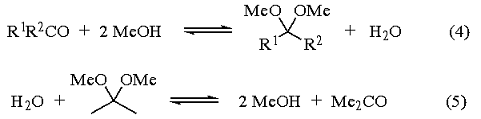
Methyl acetals of other ketones can be obtained by acidifying a mixture of the ketone, methanol, and 2,2-dimethoxypropane and removing the acetone formed by distillation. In this case, the function of the 2,2-dimethoxypropane is to react with the water formed to give methanol and acetone (eqs 4 and 5). This is evidenced by the fact that the reaction is very slow in the absence of alcohol or water, but fast when methanol is present. The rate of the reaction is also directly related to the alcohol concentration.1
One undesirable side reaction is the formation of enol ethers via the acid-catalyzed loss of one molecule of alcohol from the acetal. This occurs even at relatively low temperatures (50 °C). In order to suppress this reaction, the procedure was modified to include a neutralization with base prior to workup.
A recent example of the use of 2,2-dimethoxypropane in the above capacity involves the reaction of an aldehyde or ketone with o-Nitrobenzyl Alcohol to afford the bis-o-nitrobenzyl acetal derivative.2 This protecting group has the advantage that it is photoremovable in high yield by irradiation at 350 nm.
Acetonide Formation.
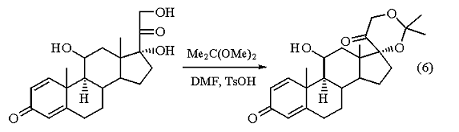
The reagent has been used to great advantage as a protecting group for the labile dihydroxyacetone side chain of the corticosteroids. Cyclic acetal formation between the 17a,21-diol grouping of the prednisolone side chain and 2,2-dimethoxypropane in an acid-catalyzed exchange reaction gives 17a,21-isopropylidenedioxypregnane in good yield (eq 6).3
he base stability of the isopropylidenedioxy function makes it useful for the preparation of C-21 modified cortical hormones whose formation requires strongly basic conditions. The acetonide is easily cleaved with aqueous acetic or formic acid in the presence of heat.
Isopropylidene Derivatives of Sugars and Nucleosides.
A mixture of 2,2-dimethoxypropane, DMF, and a catalytic amount of p-Toluenesulfonic Acid constitutes an efficient acetonating agent capable of protecting vicinal, trans-diequatorial hydroxy groups and of forming N-acetyl-2,2-dimethyloxazolidines from vicinal hydroxy and acetamido groups.4 Reaction of methyl a-D-glucopyranoside under the above conditions gives recovered starting material, methyl 4,6-O-isopropylidene-a-D-glucoside, and a trace amount of the diisopropylidene glucoside (eq 7). Similarly, deoxyinosamine, upon treatment with the same reagent mixture, affords as the major product the diisopropylidene derivative (eq 8).
A further improvement to the existing procedure involves the substitution of Tin(II) Chloride for the p-toluenesulfonic acid and 1,2-dimethoxyethane for DMF.5a Studies involving D-mannitol have shown that the tin(II) chloride does not act as a Lewis acid catalyst or as a source of HCl (by reaction with the hydroxy groups) since the use of zinc chloride under identical conditions gives no reaction. In this particular case, it was subsequently demonstrated that no catalyst is required and that isopropylidenation occurs under completely neutral conditions, presumably via acetal exchange.5b Tin(II) chloride has been used to catalyze isopropylidene formation in other carbohydrate systems.6
The ability of 2,2-dimethoxypropane to act as a water scavenger has resulted in its use (in conjunction with acetone and an acid catalyst) in the protection of the 2,3-hydroxy groups of purine and pyrimidine nucleosides.7 Here again, the formation of acetone and methanol serves to drive the equilibrium towards the product.
Esterification of Amino Acids.
Methyl ester hydrochlorides of amino acids have been prepared in which 2,2-dimethoxypropane has been used as the source of the methoxy group, the reaction solvent, and the reagent for the removal of the water formed by virtue of the hydrolysis of the acetal to methanol and acetone.8a This procedure has also been used to prepare the methyl esters of fatty acids.8b
Enol Ethers.
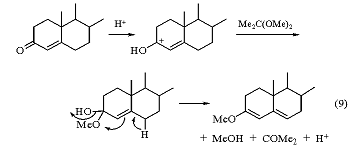
Treatment of testosterone acetate with 2,2-dimethoxypropane/DMF/TsOH converts this material into the enol ether (eq 9).9 In this case, enol ether formation is attributed to loss of water directly from the unsaturated hemiacetal.
Aldol Condensation with Enol Silyl Ethers.
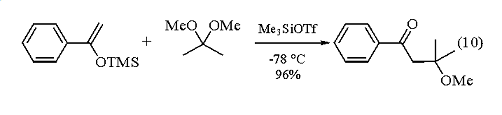
The first example of an aldol condensation between an unactivated enolate and an electrophilically activated carbonyl substrate was accomplished using enol silyl ethers and acetals or orthoesters in conjunction with a catalytic amount of Trimethylsilyl Trifluoromethanesulfonate. The reaction proceeds very readily at -78 °C and affords the erythro-aldol product with high stereoselectivity.10 The use of 2,2-dimethoxypropane in this reaction affords b-methoxy ketones (eq 10).
Hypophosphite Esters.
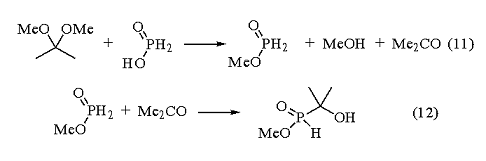
Methyl hypophosphite has been prepared by the reaction between Hypophosphorous Acid and 2,2-dimethoxypropane (eq 11).11 However, the product reacts further with the acetone formed to give 1-hydroxy-1,1-dimethylmethylphosphinate (eq 12).
Dimethyl Boronate Esters.
Dimethyl esters of arylboronic acids have been prepared by heating a mixture of the acid, 2,2-dimethoxypropane, and Zinc Chloride (catalyst).12
references
1. (a) Lorette, N. B.; Howard, W. L. JOC 1960, 25, 521, 525. (b) Brown, B. R.; MacBride, J. A. H. JCS 1964, 3822.
2. Gravel, D; Murray, S.; Ladouceur, G. CC 1985, 1828.
3. (a) Tanabe, M.; Bigley, B. JACS 1961, 83, 756. (b) Robinson, C. H.; Finckenor, L. E.; Tiberi, R.; Olivetto, E. P. JOC 1961, 26, 2863.
4. (a) de Belder, A. N. Adv. Carbohydr. Chem. Biochem 1965, 20, 219. (b) de Belder, A. N., Adv. Carbohydr. Chem. Biochem. 1977, 34, 179. (c) Evans, M. E.; Parrish, F. W.; Long, L. Carbohydr. Res 1967, 3, 453. (d) Hasegawa, A.; Fletcher, H. G. Carbohydr. Res. 1973, 29, 209, 223. (e) Hasegawa, A.; Nakajima, M. Carbohydr. Res. 1973, 29, 239.
5. (a) Chittenden, G. Carbohydr. Res. 1980, 84, 350. (b) Chittenden, G. Carbohydr. Res. 1980, 87, 219.
6. (a) Vekemans, J. A. J. M.; deBruyn, R. G. M.; Caris, R. C. H. M.; Kokx, A. J. P. M.; Konings, J. J. H. G.; Godefroi, E. F.; Chittenden, G. JOC 1987, 52, 1093. (b) Chittenden, G. RTC 1988, 107, 455.
7. (a) Hampton, A. JACS 1961, 83, 3640. (b) Moffatt, J. G. JACS 1963, 85, 1118. (c) Hampton, A. JACS 1965, 87, 4654.
8. (a) Rachele, J. R. JOC 1963, 28, 2898. (b) Radin, N. S.; Hajra, A. K.; Akahori, Y. J. Lipid Res 1960, 1, 250.
9. Nussbaum, A. L.; Yuan, E.; Dincer, D.; Olivetto, E. P. JOC 1961, 26, 3925.
10. Murata, S.; Suzuki, M.; Noyori, R. JACS 1980, 102, 3248.
11. Fitch, S. J. JACS 1964, 86, 61.
12. Matteson, D. S.; Kramer, E. JACS 1968, 90, 7261.
);You may like
See also
Lastest Price from 2,2-Dimethoxypropane manufacturers

US $0.00/KG2023-11-11
- CAS:
- 77-76-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month

US $0.00/KG2023-09-11
- CAS:
- 77-76-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg