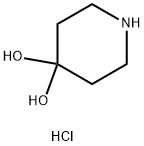
Intermediate role of 4,4-piperidinediol hydrochloride in organic synthesis
4-piperidone hydrochloride, monohydrate, also known as azinanone hydrochloride, is a derivative of piperidine used as an intermediate in manufacturing pharmaceutical drugs and chemicals [1].
The following examples show how do 4-piperidone hydrochloride work in organic synthesis.
As we know, “molecular rods” are rigid, linear macromolecules that constitute promising materials for applications in nanotechnology, such as spacers, wires, and construction elements. The poor water solubility of many current examples of molecular rods limits their applications, particularly in biology. Only a few bio-oligomers exhibit a rigid-rod structure, of which oligoprolines, which have been considered the most rigid, have been used as spacers in biochemistry. So Vincent Semetey and his colleagues studied the synthesis of water-soluble, rigid, rodlike oligopiperidines [2].
Scheme 1 outlines the strategy used to synthesize the oligopiperidines in solution. In this strategy, elongation is based on iterative reductive amination. Researches protected the commercially available 4,4-piperidinediol hydrochloride (1) with a benzyloxycarbonyl (Cbz) group to obtain 2. This compound was allowed to react with 1,4-dioxa-8-aza-spiro- [4.5]decane (3) in the presence of triacetoxyborohydride to afford piperidinopiperidin-4-one 4 diprotected with a cyclic ketal and a Cbz group. Compound 4 led to two further intermediates. Its catalytic hydrogenation yielded compound 5, and treatment of which with a concentrated aqueous HCl solution afforded compound 6. Reductive amination of 5 and 6 yielded the tetramer derivative 7. NMR and X-ray characterization results demonstrated that of tetrapiperidine 7 does not require control of the stereochemistry of the piperidine ring, but rather allows the molecule to equilibrate into the proper conformations. Because of their structure, researchers expect these molecules to be more resistant to proteolysis than polyprolines. These well-defined, water-soluble molecules may provide access to materials with well-defined structures.
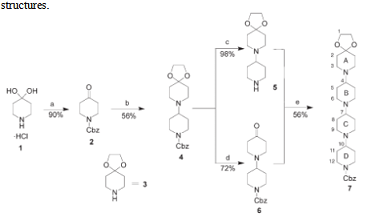
Scheme 1 Solution-phase synthesis of tetrapiperidine
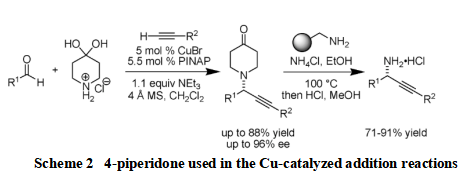
Propargylamines can serve as versatile building blocks and high-value-added intermediates for organic synthesis. However, methods that provide reliable and convenient access to propargylamines are few. Patrick Aschwanden and his colleagues documented that 4-piperidone can be used as the amine component in the Cu-catalyzed addition reactions of terminal alkynes to CdN electrophiles to provide aldimine adducts chemoselectively in useful yields and selectivities (scheme 2). The fact that 4-piperidones can be employed in the addition reaction is surprising and leads to the production of useful building blocks for asymmetric synthesis. Significantly, Patrick and team described that the substituted 4-piperidone can be readily removed, conveniently furnishing optically active primary, propargylic amines [3].
reference
[1] https://www.spectrumchemical.com/OA_HTML/chemical-synonym_4-4-Piperidinediol-Hydrochloride.jsp?pSynonym=4,4-Piperidinediol-Hydrochloride
[2] Vincent Semetey, Demetri Moustakas, and George M. Whitesides, Synthesis and Conformational Study of Water Soluble, Rigid, Rodlike Oligopiperidines, Angew. Chem. Int. Ed. 2006, 45, 588 –591.
[3] Aschwanden, Patrick; Stephenson, Corey R. J.; Carreira, Erick M., Highly Enantioselective Access to Primary Propargyl Amines: 4-Piperidinone as a Convenient Protecting Group, Organic Letters Volume8 Issue11 P2437-2440
);