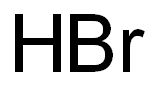Hydrobromic Acid-Hazard and Toxicity
Description
Hydrobromic Acid is a strong acid formed by dissolving the diatomic molecule HBr in water. “Constant-boiling” hydrobromic acid is an aqueous solution that distills at 124.3°C and contains 47.6% HBr by weight. Hydrobromic acid has a pKa of 9, making it a stronger acid than hydrochloric acid, but not as strong as HI, hydroiodic acid. Hydrobromic acid is one of the strongest mineral acids known.
Hydrobromic acid is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium. It is a useful reagent for generating organobromine compounds.
Certain ethers are cleaved with HBr. It also catalyzes alkylation reactions and the extraction of certain ores. Industrially significant organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis(phenol), and bromoacetic acid. Hydrobromic acid can be prepared in the laboratory via the reaction of Br2, SO2 and water. Another laboratory preparation involves the production of anhydrous HBr, which is then dissolved in water.
Toxicity Data
LD50 oral (rabbit) 900 mg/kg
LC50 inhal (rat) 2858 ppm/1 h
PEL (OSHA) 3 ppm (10 mg/m3)
TLV (ACGIH) 3 ppm (10 mg/m3; ceiling)
Major Hazards
Highly corrosive; causes severe burns on eye and skin contact and upon inhalation of gas. Hydrobromic acid and hydrogen bromide gas are highly corrosive substances that can cause severe burns upon contact with all body tissues. The aqueous acid and gas are strong eye irritants and lacrimators. Contact of concentrated hydrobromic acid or concentrated HBr vapor with the eyes may cause severe injury, resulting in permanent impairment of vision and possible blindness.
Skin contact with the acid or HBr gas can produce severe burns. Ingestion can lead to severe burns of the mouth, throat, and gastrointestinal system and can be fatal. Inhalation of hydrogen bromide gas can cause extreme irritation and injury to the upper respiratory tract and lungs, and exposure to high concentrations may cause death. HBr gas is regarded as having adequate warning properties.
Toxicity
Hydrobromic acid and hydrogen bromide gas are highly corrosive substances that can cause severe burns upon contact with all body tissues. The aqueous acid and gas are strong eye irritants and lacrimators. Contact of concentrated hydrobromic acid or concentrated HBr vapor with the eyes may cause severe injury, resulting in permanent impairment of vision and possible blindness.
Skin contact with the acid or HBr gas can produce severe burns. Ingestion can lead to severe burns of the mouth, throat, and gastrointestinal system and can be fatal. Inhalation of hydrogen bromide gas can cause extreme irritation and injury to the upper respiratory tract and lungs, and exposure to high concentrations may cause death. HBr gas is regarded as having adequate warning properties. Hydrogen bromide has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans.
Flammability and Explosibility
Noncombustible, but contact with metals may produce highly flammable hydrogen gas.
Reactivity and Incompatibility
Hydrobromic acid and hydrogen bromide react violently with many metals with the generation of highly flammable hydrogen gas, which may explode. Reaction with oxidizers such as permanganates, chlorates, chlorites, and hypochlorites may produce chlorine or bromine.
Storage and Handling
Splash goggles and rubber gloves should be worn when handling this acid, and containers of HBr should be stored in a wellventilated location separated from incompatible metals. Water should never be added to HBr because splattering may result; always add acid to water. Containers of hydrobromic acid should be stored in secondary plastic trays to avoid corrosion of metal storage shelves due to drips or spills. Cylinders of hydrogen bromide should be stored in cool, dry locations, separated from alkali metals and other incompatible substances.
Accidents
In the event of skin contact, remove contaminated clothing and immediately wash with flowing water for at least 15 min. In case of eye contact, immediately wash with copious amounts of water for at least 15 min while holding the eyelids open. Seek medical attention. In case of ingestion, do not induce vomiting. Give large amounts of water or milk if available and transport to medical facility. In case of inhalation, remove to fresh air and seek medical attention.
Carefully neutralize spills of hydrobromic acid with a suitable agent such as powdered sodium bicarbonate, further dilute with absorbent material, place in an appropriate container, and dispose of properly. Dilution with water before applying the solid adsorbent may be an effective means of reducing exposure to hydrogen bromide vapor. Respiratory protection may be necessary in the event of a large spill or release in a confined area.
Leaks of HBr gas are evident from the formation of dense white fumes on contact with the atmosphere. Small leaks can be detected by holding an open container of concentrated ammonium hydroxide near the site of the suspected leak; dense white fumes confirm a leak is present. In case of the accidental release of hydrogen bromide gas, such as from a leaking cylinder or associated apparatus, evacuate the area and eliminate the source of the leak if this can be done safely. Remove cylinder to a fume hood or remote area if it cannot be shut off. Full respiratory protection and protective clothing may be required to deal with a hydrogen bromide release.
Disposal
In many localities, hydrobromic acid or the residue from a spill may be disposed of down the drain after appropriate dilution and neutralization. Otherwise, hydrobromic acid and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines. Excess hydrogen bromide in cylinders should be returned to the manufacturer.
);You may like
See also
Lastest Price from Hydrogen bromide manufacturers

US $10.00-30.00/kg2024-04-24
- CAS:
- 10035-10-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100ton/ month

US $0.00-0.00/kg2023-12-11
- CAS:
- 10035-10-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons