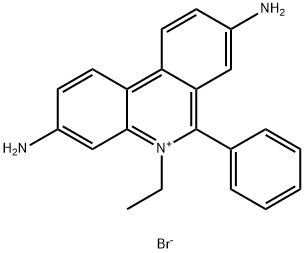
Ethidium bromide-Hazard and Toxicity
Description
Ethidium bromide (abbreviated EB) is common fluorescent dyes of observing DNA under ultraviolet ray, it belongs intercalating dye because of its polycyclic structure, which enables it to be inserted between nucleotide bases. Since the fluorescence quantum yield of nucleic acids and bases is very low, so that the use of natural fluorescence detection of nucleic acids is more difficult than protein, the fluorescent probe technique in nucleic acid research is particularly important.
Ethidium bromide (et-hidium bromide or called ethidium bromide) is used commonly, which has the specifical ability of bond with nucleic acid (DNA, RNA, double-stranded polynucleotide). The minimum detectable amount of desoxyribonucleic acid up to 10ng/ml, and the combination with double-stranded region of the nucleic acid is single-minded, it can distinguish between various configurations nucleic acid by the change of fluorescence intensity which producted by different proportions of the various conformation of nucleic acids.
Major Hazards
Potent mutagen. Acute toxic effects from exposure to ethidium bromide have not been thoroughly investigated. Ethidium bromide is irritating to the eyes, skin, mucous membranes, and upper respiratory tract. Although there is no evidence for the carcinogenicity or teratogenicity of this substance in humans, ethidium bromide is strongly mutagenic and therefore should be regarded as a possible carcinogen and reproductive toxin.
Toxicity
Acute toxic effects from exposure to ethidium bromide have not been thoroughly investigated. Ethidium bromide is irritating to the eyes, skin, mucous membranes, and upper respiratory tract.
Although there is no evidence for the carcinogenicity or teratogenicity of this substance in humans, ethidium bromide is strongly mutagenic and therefore should be regarded as a possible carcinogen and reproductive toxin.
Flammability and Explosibility
Ethidium bromide does not pose a flammability hazard (NFPA rating = 1).
Storage and Handling
Ethidium bromide should be handled in the laboratory using the "basic prudent practices". Because of its mutagenicity, stock solutions of this compound should be prepared in a fume hood, and protective gloves should be worn at all times while handling this substance. Operations capable of generating ethidium bromide dust or aerosols of ethidium bromide solutions should be conducted in a fume hood to prevent exposure by inhalation.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If ethidium bromide is ingested, obtain medical attention immediately. In the event of a spill, mix ethidium bromide with an absorbent material (avoid raising dust), place in an appropriate container, and dispose of properly. Soak up aqueous solutions with a spill pillow or absorbent material.
Disposal
Excess ethidium bromide and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
);You may like
Related articles And Qustion
Lastest Price from Ethidium bromide manufacturers
US $8.00-1.00/kg2024-04-05
- CAS:
- 1239-45-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $583.00/g2023-07-10
- CAS:
- 1239-45-8
- Min. Order:
- 25g
- Purity:
- 95%
- Supply Ability:
- 1000kg