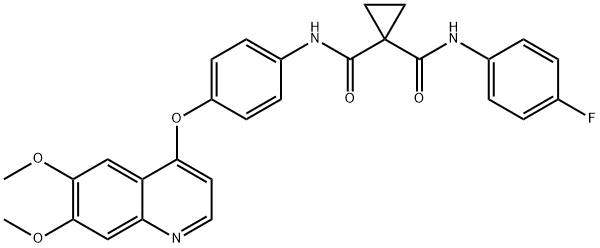
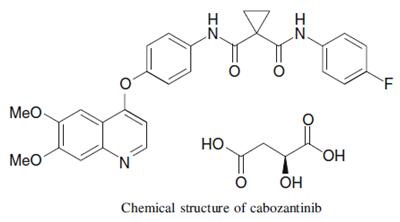
Cabozantinib (XL184)- antitumour effects
Cabozantinib is a small molecule inhibitor of multiple tyrosine kinase receptors. In vitro, it has demonstrated inhibitory activity against RET, MET and VEGFR-2 and against the RET mutant M918T. Cabozantinib inhibited the (auto)phosphorylation of MET, RET and VEGFR-2 in vitro and in murine tumour models, and the proliferation of cells expressing the MEN 2A mutation or the RET mutant C634 W. It also inhibited hepatocyte growth factor-stimulated migration and invasion, and VEGFinduced endothelial cell tubule formation in vitro. In a murine xenograft model of breast cancer, cabozantinib induced tumour hypoxia and apoptosis, and reduced proliferation and vascularity, inducing tumour and endothelial cell death [1-2].
Antitumour activity with cabozantinib has been demonstrated in various murine xenograft tumour models (breast cancer, lung cancer, MTC and prostate cancer). For instance, in the murine xenograft MTC model, significant tumour suppression was observed following oral cabozantinib 10, 30 and 60 mg/kg once daily compared with vehicle, with the 10 and 30 mg/kg once daily dosages suppressing tumour growth in a dosedependent manner. Moreover, an analysis of tumour cellularity demonstrated significant tumour regression (defined as a reduction from the start of therapy to the final measurement in mean tumour weight) following cabozantinib 10, 30 and 60 mg/kg, but not 3 mg/kg once daily versus vehicle [3].
Inhibitors that target VEGFR-2 are known to promote metastasis; this has been demonstrated in preclinical models. This may be the result of increased signaling via MET, with the simultaneous targeting of MET and VEGFR-2 potentially assisting in the inhibition of alternative pathway signalling and, thus, providing a more sustained antitumour effect. In a murine xenograft model of breast cancer, treatment with cabozantinib did not promote metastasis, with no differences observed in lung surface tumour burden, number of foci or whole lung wet weights compared with vehicle [4].
The antitumour effects of cabozantinib have also been observed in patients with solid tumours participating in phase I and II studies. Patients in a phase I, dose escalation study received cabozantinib administered intermittently (once daily for the first 5 days of a 2-week period) at doses ranging from 0.08 to 11.52 mg/kg or continuously (once daily) at doses ranging from 175 to 265 mg. Of the 35 patients who had measurable MTC in this study, 49 % experienced at least a 30 % reduction from baseline in tumour measurements and 29 % achieved an objective response (all objective responses were partial responses). In a subsequent analysis conducted after a minimum follow-up duration of 52 months, 11 (30 %) of 37 MTC patients achieved a confirmed partial response or were progression free at ≥ 24 months; all 11 patients were progression free for ≥ 24 months. Five of these 11 patients remained on treatment at the time of data cut-off, with a median duration of treatment of 55 months, with four of these patients having achieved a partial response and one stable disease at this timepoint [5-6].
In all, current evidence suggests cabozantinib to be a valuable treatment option for adults with progressive, unresectable locally advanced or metastatic MTC.
References
1.Bentzien F, Zuzow M, Heald N. In vitro and in vivo activity of cabozantinib (XL184), an inhibitor of RET, MET, and VEGFR2, in a model of medullary thyroid cancer[J]. Thyroid. 2013,23(12):1569–77.
2.Yakes FM, Chen J, Tan J. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer[J]. Ther. 2011, 10(12):2298–308.
3.Graham TJ, Box G, Tunariu N. Preclinical evaluation of imaging biomarkers for prostate cancer bone metastasis and response to cabozantinib[J]. J Natl Cancer Inst. 2014, 106(4):dju033.
4.Nagilla M, Brown RL, Cohen EEW. Cabozantinib for the treatment of advanced medullary thyroid cancer[J]. Adv Ther. 2012, 29(11):925–34.
5.Kurzrock R, Sherman SI, Ball DW. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer[J]. J Clin Oncol. 2011, 29(19):2660–6.
6.Cohen EEW, Hong DS, Pfister DG. Long-term disease control of C2 years achieved with cabozantinib in subjects with metastatic medullary thyroid carcinoma on a phase I study [J]. J Clin Oncol. 2013, 31:15 Suppl. 1.
You may like
Related articles And Qustion
See also
Lastest Price from Cabozantinib manufacturers
![849217-68-1 N-[4-[(6,7-Dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide](/ProductImageEN/2023-09/Small/b566bf0d-fda0-4dff-ba87-e69f67574627.png)
US $0.00/kg2024-04-10
- CAS:
- 849217-68-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 500kg

US $0.00/g2024-03-12
- CAS:
- 849217-68-1
- Min. Order:
- 1g
- Purity:
- 98% HPLC
- Supply Ability:
- 1kg