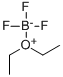Boron trifluoride etherate--Chemical properties & Reactivity
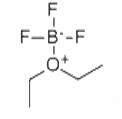
Boron trifluoride etherate, strictly boron trifluoride diethyl etherate, or boron trifluoride–ether complex, is the chemical compound with the formula BF3O(C2H5)2, often abbreviated BF3OEt2. It is a colorless liquid, although older samples can appear brown. With the flash point between 37.8 and 93.3 °C, BF3OEt2 was chemically active. BF3OEt2 could undergoes violent chemical change at elevated temperatures and pressures, reacts violently with water, or may form explosive mixtures with water. It reacts exothermically with water to form extremely flammable diethyl ether and toxic, corrosive boron trifluoride hydrates. Immediately hydrolyzed by moisture in air to form hydrogen fluoride, BF3OEt2 is a fuming liquid with a sharp pungent odour. Upon exposure to water BF3OEt2 may emit flammable and corrosive vapors. BF3OEt2 may be corrosive to skin, eyes and mucous membranes. Short exposure could cause serious temporary or moderate residual injury. BF3OEt2 may be toxic by inhalation. The chemical is incompatible with bases, amines, and alkali metals.
The compound is used as a source of boron trifluoride in many chemical reactions that require a Lewis acid. The compound features tetrahedral boron coordinated to a diethylether ligand. Many analogues are known, including the methanol complex. Traditionally used as a catalyst of organic synthesis, BF3OEt2 was frequently-used in acetylation, alkylation, polymerization, dehydration and condensation reaction. BF3OEt2 may also be a basic ingredient of producing high quality borohydride fuel and extracting the isotope of boron. Not only used as a catalyst for polybutadiene, polyoxymethylene, coumarone, paints, etc., BF3OEt2 was also a curing agent for epoxy resin. Besides, BF3OEt2 was a common analytical reagent.
Produced by heating the sulfuric acid, calcium fluoride (fluorite) and boric acid together, boron trifluoride gas reacts with ether boron to produce the trifluoride etherate crude product. The crude complex compound could be obtained by vacuum distillation. The consumption of raw material is as followed: boric acid (≥98%), 560kg/t; calcium fluoride(≥90%) 1150kg/t; fuming sulfuric acid (104.5%), 4100kg/t; ether(≥99%) 725kg/t. Refining would be conducted to get the finished product.
References
[1] Qin, K.-Y.; Su, G.-F.; Rao, W.-P.; Tan, G.-M. Boron trifluoride etherate in organic synthesis, MOJ Biorg. Org. Chem., 2019;3(1):1‒9.
[2] Nie, G.; Yang, H.; Wang, S.; Li, X. High-Quality Inherently Organic Conducting Polymers Electrosynthesized from Fused-Ring Compounds in a New Electrolytic System Based on Boron Trifluoride Diethyl Etherate, Crit. Rev. Solid State, 2011, 36(4): 209-228.
You may like
See also
Lastest Price from Boron trifluoride diethyl etherate manufacturers

US $0.00-0.00/ml2023-11-27
- CAS:
- 109-63-7
- Min. Order:
- 100ml
- Purity:
- 99%
- Supply Ability:
- 60L

US $0.00/KG2023-10-09
- CAS:
- 109-63-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month