Benzyl Chloride: A Potential Hazardous Chemical
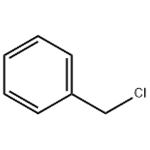
Benzyl chloride or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. The colourless liquid is a reactive organochlorine compound that is an extensively used chemical building block. Benzyl chloride is made use as a chemical intermediate to manufacture certain dyes and pharmaceutical products, plasticizer, flavorants, and perfumes and as a photographic developer. Below are the some of the applications of Benzyl Chloride in chemical industry:
1. Plasticizers
2. Surfactants
3. Flavor and Fragrance
4. Sanitizing agent
5. Pharmaceuticals
6. Dyes
7. Fuels and additives
8. Paint and coating additives

Production of Benzyl Chloride
Benzyl Chloride is produced by the thermal or photochemical chlorination of toluene under 65-100 deg Celsius. On lower temperatures the quantity of ring-chlorinated by-products is greater. The chlorination is usually carried to barely about 50 percent of toluene conversion so as to reduce the quantity of benzal chloride formed. When toluene is recycled and the over chlorinated products are separated by the process of distillation, overall yield based on toluene is more than 90%.
Distinct materials, comprising the phosphorus pentachloride, have been accounted to catalyze the side-chain chlorination. These compounds and others such as amides also reduce ring chlorination by complex metallic impurities. In commercial practice, chlorination may be carried out either batch wise or continuously.
As a Hazard Chemical
Benzyl chloride gets hydrolysed into benzyl alcohol and hydrochloric acid when it is exposed to water. Hence, benzyl chloride is deliberated as a lachrymator agent or a chemical that is cause for eye, respiratory, and skin irritation. One of the main effects of benzyl chloride inhalation includes acute irritation of the skin, eyes, and mucous membranes, which can even result in lung damage. The nervous system can be damaged because of the exposure to excessive concentration of the chemical. Benzyl chloride was initially used for the purpose of a chemical weapon in the course of World War I.
Benzyl chloride burns but does not ignite readily, whereas it ignites combustibles. When heated to decomposition, benzyl chloride releases toxic and corrosive fumes. Certain organic chlorides decompose to yield phosgene. Benzyl chloride is incompatible with metals for instance copper, aluminum, magnesium, iron, zinc, and tin. Benzyl chloride needs to kept separate from oxidizing materials. Benzyl chloride reacts with water resulting in some non-violent release of energy.
);You may like
Related articles And Qustion
See also
Lastest Price from Benzyl chloride manufacturers

US $10.00-30.00/kg2024-03-29
- CAS:
- 100-44-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100ton/ month
US $100.00-1.00/KG2024-03-25
- CAS:
- 100-44-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available



