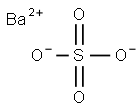Barium sulfate – a widely used Metal Sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula BaSO4. It is a barium salt and a metal sulfate. It is a white crystalline solid that is odorless and virtually insoluble in water at room temperature. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it. it is mostly used as a component in oil well drilling fluid it occurs naturally as the mineral barite. It has a role as a radio opaque medium. The white opaque appearance and its high density are exploited in its main applications [1,2].
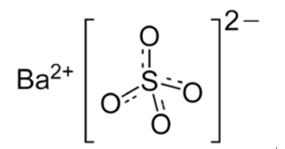
Barium sulfate is reduced to barium sulfide by carbon. The accidental discovery of this conversion many centuries ago led to the discovery of the first synthetic phosphor [2]. The sulfide, unlike the sulfate, is water-soluble. During the early part of the 20th century, during the Japanese colonization period, hokutolite was found to exist naturally in the Beitou hot-springs area near Taipei City, Taiwan. Hokutolite is a radioactive mineral composed mostly of PbSO4 and BaSO4, but also containing traces of U, Th and Ra. The Japanese harvested these elements for industrial uses, and also developed dozens of “therapeutic hot-spring baths” in the area [3].
Almost all of the barium consumed commercially is obtained from barite, which is often highly impure. Barite is processed by carbothermal reduction (heating with coke) to give barium sulfide:
BaSO4 + 4 C → BaS + 4 CO
In contrast to barium sulfate, barium sulfide is soluble in water and readily converted to the oxide, carbonate, and halides. To produce highly pure barium sulfate, the sulfide or chloride is treated with sulfuric acid or sulfate salts:
BaS + H2SO4 → BaSO4 + H2S
Barium sulfate produced in this way is often called blanc fixe, which is French for "permanent white." Blanc fixe is the form of barium encountered in consumer products, such as paints [4].
In the laboratory barium sulfate is generated by combining solutions of barium ions and sulfate salts. Because barium sulfate is the least toxic salt of barium due to its insolubility, wastes containing barium salts are sometimes treated with sodium sulfate to immobilize (detoxify) the barium. Barium sulfate is one of the most insoluble salts of sulfate. Its low solubility is exploited in qualitative inorganic analysis as a test for Ba2+ ions as well as for sulfate.
Although soluble salts of barium are moderately toxic to humans, barium sulfate is nontoxic due to its insolubility. The most common means of inadvertent barium poisoning arises from the consumption of soluble barium salts mislabeled as BaSO4. In the Celobar incident (Brazil, 2003), nine patients died from improperly prepared radiocontrast agent. In regards to occupational exposures, the Occupational Safety and Health Administration set a permissible exposure limit at 15 mg/m3 while the National Institute for Occupational Safety and Health has a recommended exposure limit at 10 mg/m3. For respiratory exposures, both agencies have set an occupational exposure limit at 5 mg/m3 [5].
Barium sulfate can be used as a weighting mud in oil-drilling, in paints, paper coatings, linoleum, textiles, rubber, etc.
For drilling fluids, about 80% of the world's barium sulfate production, mostly purified mineral, is consumed as a component of oil well drilling fluid. It increases the density of the fluid [4], increasing the hydrostatic pressure in the well and reducing the chance of a blowout.
For radiocontrast agent, barium sulfate in suspension is frequently used medically as a radiocontrast agent for X-ray imaging and other diagnostic procedures. It is most often used in imaging of the GI tract during what is colloquially known as a "barium meal". It is administered orally, or by enema, as a suspension of fine particles in a thick milk like solution (often with sweetening and flavoring agents added). Although barium is a heavy metal, and its water-soluble compounds are often highly toxic, the low solubility of barium sulfate protects the patient from absorbing harmful amounts of the metal. Barium sulfate is also readily removed from the body, unlike Thorotrast, which it replaced. Due to the relatively high atomic number (Z = 56) of barium, its compounds absorb X-rays more strongly than compounds derived from lighter nuclei.
For pigment, the majority of synthetic barium sulfate is used as a component of white pigment for paints. In oil paint, barium sulfate is almost transparent, and is used as a filler or to modify consistency. One major manufacturer of artists' oil paint sells "permanent white" that contains a mixture of titanium white pigment (TiO2) and barium sulfate. The combination of barium sulfate and zinc sulfide (ZnS) is the inorganic pigment called lithopone. In photography it is used as a coating for certain photographic papers [4].
For paper brightener, a thin layer of barium sulfate called baryta is first coated on the base surface of most photographic paper to increase the reflectiveness of the image, with the first such paper introduced in 1884 in Germany. The light-sensitive silver halide emulsion is then coated over the baryta layer. The baryta coating limits the penetration of the emulsion into the fibers of the paper and makes the emulsion more even, resulting in more uniform blacks. (Further coatings may then be present for fixing and protection of the image.) More recently, baryta has been used to brighten papers intended for ink-jet printing [1].
Moreover, barium sulfate can be used in plastics filler, soil tester, catalyst support, pyrotechnics, copper industry, Radiometric measurements [1].
References
[1] https://en.wikipedia.org/wiki/Barium_sulfate
[2] Holleman, A. F. and Wiberg, E. (2001) Inorganic Chemistry, San Diego, CA : Academic Press, ISBN 0-12-352651-5
[3] Chu, Tieh-Chi; Wang, Jeng-Jong (2000). "Radioactive Disequilibrium of Uranium and Thorium Nuclide Series in Hot Spring and River Water from Peitou Hot Spring Basin in Taipei". Journal of Nuclear and Radiochemical Sciences. 1 (1): 5–10. doi:10.14494/jnrs2000.1.5.
[4] Robert Kresse, Ulrich Baudis, Paul Jäger, H. Hermann Riechers, Heinz Wagner, Jochen Winkler, Hans Uwe Wolf, "Barium and Barium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2007 Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_325.pub2
[5] "Barium Sulfate". NIOSH Pocket Guide to Chemical Hazards. Centers for Disease Control and Prevention. April 4, 2011. Retrieved November 18, 2013.
You may like
Related articles And Qustion
See also
Lastest Price from Barium sulfate manufacturers

US $5.00-3.50/grams2024-04-25
- CAS:
- 7727-43-7
- Min. Order:
- 1grams
- Purity:
- 99%
- Supply Ability:
- 100Tons

US $6.00/KG2024-04-23
- CAS:
- 7727-43-7
- Min. Order:
- 1KG
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/Month