Applications of N-Nitrosodimethylamine
N-Nitrosodimethylamine(NDMA) is a nitrosamine, and it plays a role as a mutagen. N-nitrosodimethylamine is a yellow oily liquid with a faint characteristic odor. Boiling point 151-153°C. N-nitrosodimethylamine can reasonably be expected to be a carcinogen. N-nitrosodimethylamine also is used as an antioxidant, as an additive for lubricants and as a softener of copolymers. N-Nitrosodimethylamine, also known as dimethylnitrosamine (DMN), is a semi-volatile organic chemical, produced as by-product of several industrial processes and present at very low levels in certain foodstuffs, especially those cooked, smoked, or cured. N-Nitrosodimethylamine is water-soluble, yellow in color, and has little or no taste and odor. N-Nitrosodimethylamine is toxic to the liver and other organs and is a probable human carcinogen. N-Nitrosodimethylamine is also used to create cancer in rats for cancer research.
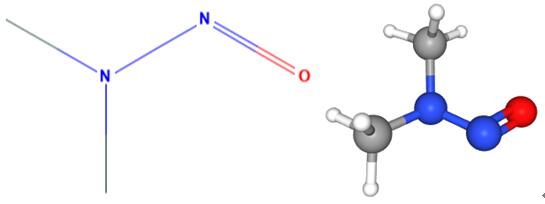
Fig 1. Chemical structure formula and three-dimensional structure of N-Nitrosodimethylamine
N-Nitrosodimethylamine is an industrial by-product or waste product of several industrial processes, such as manufacturing of unsymmetrical dimethylhydrazine (UDMH), which is a component of rocket fuel that requires N-Nitrosodimethylamine for its synthesis. Of more general concern, water treatment by chlorination or chloramination of organic nitrogen-containing wastewater can lead to the production of N-Nitrosodimethylamine at potentially harmful levels[1]. Further, N-Nitrosodimethylamine can form or be leached during treatment of water by anion exchange resins. Finally, N-Nitrosodimethylamine is found at low levels in numerous items of human consumption, including cured meat, fish, beer, tobacco smoke[2] and as an impurity in pharmaceuticals. N-Nitrosodimethylamine is, however, unlikely to bioaccumulate.
N-Nitrosodimethylamine has been used as an intermediate in the manufacture of 1,1-dimethylhydrazine, which is a component of rocket propellants. In the workplace, N-Nitrosodimethylamine also has been encountered as a contaminant of common organic amines or as a reaction by-product. Exposures have been possible in cancer research laboratories where the material may be excreted unchanged by laboratory animals.
N-Nitrosodimethylamine is primarily used as a research chemical. N-Nitrosodimethylamine has been used as an antioxidant, as an additive for lubricants, and as a softener of copolymers. N-Nitrosodimethylamine was formerly used in the production of rocket fuels[3,4].
N-Nitrosodimethylamine has been found to be carcinogenic in a number of animal species, inducing tumors in various organs and by various routes of exposure. Increased incidences of liver, kidney, and lung tumors have been observed in rats and mice that inhaled N-nitrosodimethylamine. Liver tumors have also been
observed in orally exposed rats, mice, and hamsters[5].
References
[1] David L. Sedlak, Rula A. Deeb, Elisabeth L. Hawley, William A. Mitch, Timothy D. Durbin, Sam Mowbray and Steve Carr (2005). "Sources and Fate of Nitrosodimethylamine and Its Precursors in Municipal Wastewater Treatment Plants". Water Environment Research. 77 (1, Emerging Micropollutants in Treatment Systems (Jan.–Feb. 2005)): 32–39.
[2] Najm, I.; Trussell, R. R. (2001). "NDMA Formation in Water and Wastewater". Journal American Water Works Association. 93 (2): 92–99.
[3] U.S. Department of Health and Human Services. Hazardous Substances Data Bank (HSDB, online database).National Toxicology Information Program, National Library of Medicine, Bethesda, MD. 1993.
[4] The Merck Index. An Encyclopedia of Chemicals, Drugs, and Biologicals. 11th ed. Ed. S. Budavari. Merck and Co. Inc., Rahway, NJ. 1989.
[5] Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for NNitrosodimethylamine.Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA.1989.