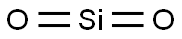Application of Quartz
General description
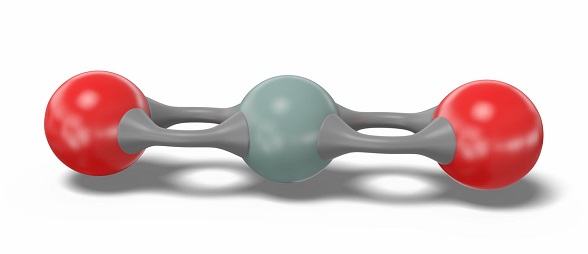
Quartz (Silicon Dioxide) is a natural compound of silicon and oxygen found mostly in sand, Silica has three main crystalline varieties: quartz, tridymite, and cristobalite. Fine particulate silica dust from quartz rock causes over a long-term progressive lung injury, silicosis. (NCI04) Silica is another name for the chemical compound composed of silicon and oxygen with the chemical formula SiO2, or silicon dioxide. There are many forms of silica. All silica forms are identical in chemical composition, but have different atom arrangements. Silica compounds can be divided into two groups, crystalline (or c-silica) and amorphous silica (a-silica or non-crystalline silica). c-Silica compounds have structures with repeating patterns of silicon and oxygen. a-Silica chemical structures are more randomly linked when compared to c-silica. All forms of silica are odorless solids composed of silicon and oxygen atoms. Silica particles become suspended in air and form non-explosive dusts. Silica may combine with other metallic elements and oxides to form silicates. Silicon dioxide is a silicon oxide made up of linear triatomic molecules in which a silicon atom is covalently bonded to two oxygens.
Application
The general name of quartz and its various variants. Its basic structural unit is silicon oxygen tetrahedron, which has high temperature resistance, corrosion resistance, low coefficient of thermal expansion, high insulation and unique optical properties [1]. High purity quartz is generally obtained from natural quartz ore after processing and purification, and a small amount is synthesized artificially. The SiO2 content is greater than 99 9%, which has important applications in key fields such as optical fiber communication, photovoltaic, electronics and national defense. It is a world recognized strategic key mineral resource. In the context of new energy and carbon neutralization, various emerging technologies and industries have been strongly supported and developed, and high-purity quartz materials have also received a lot of attention. the development of quartz-enhanced photoacoustic sensors (QEPAS) for the sensitive and selective quantification of molecular trace gas species with resolved spectroscopic features is reported. The basis of the QEPAS technique, the technology available to support this field in terms of key components, such as light sources and quartz-tuning forks and the recent developments in detection methods and performance limitations will be discussed. Furthermore, different experimental QEPAS methods such as: on-beam and off-beam QEPAS, quartz-enhanced evanescent wave photoacoustic detection, modulation-cancellation approach and mid-IR single mode fiber-coupled sensor systems will be reviewed and analysed. A QEPAS sensor operating in the THz range, employing a custom-made quartz-tuning fork and a THz quantum cascade laser will be also described[1].
The quartz crystal microbalance (QCM) is a highly sensitive instrument which determines the nature of binding interactions in real time within a label free environment. Current advances in this technology have afforded increased stability, sensitivity, parallel control availability, high through-put capacity and an increased range of experimental configurations. QCM is ideally suited to the study of nanomedicine where investigation of nanovehicle performance in the biological milieu has become of increased clinical significance. Primary issues include activation of the complement system and induction of apoptosis. It is the aim of this review to discuss the opportunities afforded by the QCM in the mechanistic understanding of interaction in the biological environment and how this can be applied to improved nanoconstruct design to attain performance enhancement[2].
Aqueous solubility of an active pharmaceutical ingredient is an important consideration to ensure successful drug development. Mesoporous materials have been investigated as an amorphous drug delivery system owing to their nanosized capillaries and large surface areas. The complex interactions of crystalline compounds with mesoporous media and their implication in drug delivery are not well understood. Molecules interacting with porous media behave very differently than those in bulk phase. Their altered dynamics and thermodynamics play an important role in the properties and product performance of the amorphous system. This application of mesoporous silicon dioxide and silicates in drug amorphization is the main focus[3].
Synthesis
With the development of global science and technology and the improvement of application demand in the field of high and new technology, the development and development of emerging industries have received special attention, among which the development and application of quartz mineral resources have also received great attention from all countries. In view of the development and application status and technical problems of quartz mineral resources, this paper summarizes the types, reserves and consumption structure of quartz in China, expounds the impurity types of quartz and the purification and synthesis technology of quartz, and analyzes in detail the application of high-purity quartz in semiconductor industry, optical fiber industry, photovoltaic industry, optical field This paper introduces the application status and technical difficulties in strategic emerging industries such as aerospace, and expounds the key influence and control methods of impurities on product performance in these fields[4].
There are three kinds of preparation methods of high-purity quartz: direct processing of natural crystal, purification of quartz minerals with low impurities and chemical synthesis of silicon containing compounds. There are few natural crystal mineral resources. In order to change and get rid of the dependence on crystal resources, the method of directly purifying unnatural quartz to obtain high-purity quartz has been studied at home and abroad. At present, only a few developed countries such as Europe, America, Russia and Japan can achieve it. In the 1990s, Japan successfully purified high-purity quartz from pegmatite, Germany and Russia also purified high-purity quartz from vein quartz and quartzite respectively [4]. In the 1980s, American PPCC company purified high-purity quartz (99.99%) from granite in foxdale area on the northwest coast of Britain. The purification process of high-purity quartz first breaks and grinds the vein quartz or quartzite to the required particle size and removes some impurities, and then separates or dissolves the impurities by physical and chemical means. The whole purification process can be simply summarized into three processes: pretreatment, physical treatment and chemical treatment. Specifically, various beneficiation methods such as crushing, grinding, screening, magnetic separation, pickling, chlorination roasting and so on are adopted. According to the composition and grade of raw ore, the corresponding purification process of quartz is designed: quartz ore with high sodium content needs high-temperature calcination, and carbonate minerals with high calcium and magnesium need hydrochloric acid pretreatment.
Figure 1 ( a) Typical isomorphic substitution in quartz crystal; ( b) Theoretical configuration of atomic micro - cluster
With the development of emerging industries in recent years, such as new energy, semiconductor, aerospace and other fields, the demand for high-purity quartz has also increased greatly. Different fields need quartz with different purity. Although China is rich in quartz mineral resources, due to the limitations of production cost and purification process, the supply capacity of high-purity quartz is weak and the import volume is large. Therefore, while strengthening the exploration of quartz mineral resources, we should improve quartz purification technology and increase the output of high-purity quartz to meet the needs of China's new energy, new materials and high-precision fields. The key reason for the relatively high cost and stable application of high-temperature chlorination process and chemical roasting process in China is the lack of professional raw ore pre-treatment equipment. The research on the purification of high-purity quartz in China is mainly in the process, and there is less theoretical research. Only when the theoretical form and purification process of quartz are combined with the actual type of impurities, can the purification process of quartz be optimized to the maximum extent. Behind all this, we need corresponding supporting human resources, processing equipment and quality testing equipment. Therefore, we should mobilize and organize scientific research forces at the national level to vigorously support the research and development of high-end quartz products in China.
Reference
1.Hunter A. C., "Application of the Quartz Crystal Microbalance to Nanomedicine," Journal of Biomedical Nanotechnology, Vol.5, No.6(2009), pp.669-675.
2.Patimisco P., Scamarcio G. & Tittel F. et al., "Quartz-Enhanced Photoacoustic Spectroscopy: A Review," Sensors, Vol.14, No.4(2014), pp.6165-6206.
3.Qian K. K. & Bogner R. H., "Application of mesoporous silicon dioxide and silicate in oral amorphous drug delivery systems," J Pharm Sci, Vol.101, No.2(2012), pp.444-463.
4.Ouyangjing, Chen Guang, Liang Lixing, etc.: Analysis on Purification of quartz mineral resources and application technology in strategic emerging industries, mineral protection and utilization, No. 06, 2021, pp. 35-45.
);Related articles And Qustion
Lastest Price from Quartz manufacturers

US $18.00-15.00/kg2024-04-19
- CAS:
- 14808-60-7
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 100Tons

US $100.00/kg2024-02-27
- CAS:
- 14808-60-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100 tons