Acetonitrile- The Simplest Organic Nitrile
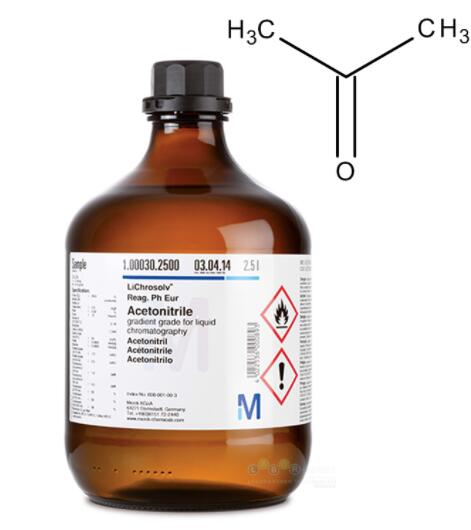
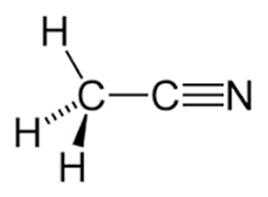
Acetonitrile is a nitrile that is hydrogen cyanide in which the hydrogen has been replaced by a methyl group. It is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It has a role as a polar aprotic solvent and an EC 3.5.1.4 (amidase) inhibitor. It is an aliphatic nitrile and a volatile organic compound with the formula CH3CN. Acetonitrile appears as a colorless limpid liquid with an aromatic odor. Flash point 42°F. Density 0.783 g/cm3. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas. Toxic by skin absorption. Less dense than water. Vapors are denser than air. It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene [1].
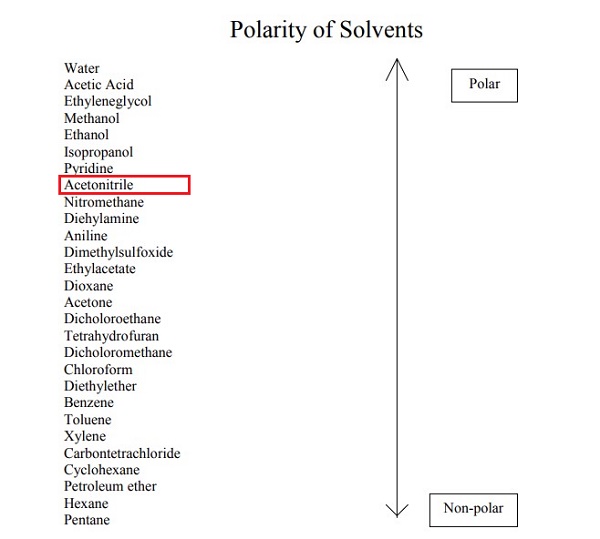
Acetonitrile is completely miscible with water, and its high dielectric constant and dipole moment make it a suitable solvent for many inorganic and organic compounds. Most polar organic materials are soluble in Acetonitrile.

In the laboratory, it is used as a medium-polarity solvent that is miscible with water and a range of organic solvents, but not saturated hydrocarbons. It has a convenient liquid range and a high dielectric constant of 38.8. With a dipole moment of 3.92 D, acetonitrile dissolves a wide range of ionic and nonpolar compounds and is useful as a mobile phase in HPLC and LC–MS. The N≡C−C skeleton is linear with a short C≡N distance of 1.16 Å [2].
Acetonitrile is a byproduct from the manufacture of acrylonitrile. Most is combusted to support the intended process but an estimated several thousand tons are retained for the above-mentioned applications. Production trends for acetonitrile thus generally follow those of acrylonitrile. Acetonitrile can also be produced by many other methods, but these are of no commercial importance as of 2002. Illustrative routes are by dehydration of acetamide or by hydrogenation of mixtures of carbon monoxide and ammonia. In 1992, 14,700 tonnes (32,400,000 lb) of acetonitrile were produced in the US. Catalytic ammoxidation of ethylene was also researched [3].
Starting in October 2008, the worldwide supply of acetonitrile was low because Chinese production was shut down for the Olympics. Furthermore, a U.S. factory was damaged in Texas during Hurricane Ike [4]. Due to the global economic slowdown, the production of acrylonitrile that is used in acrylic fibers and acrylonitrile butadiene styrene (ABS) resins decreased. Acetonitrile is a byproduct in the production of acrylonitrile and its production also decreased, further compounding the acetonitrile shortage. The global shortage of acetonitrile continued through early 2009.
Acetonitrile has only modest toxicity in small doses. It can be metabolised to produce hydrogen cyanide, which is the source of the observed toxic effects [5]. Generally the onset of toxic effects is delayed, due to the time required for the body to metabolize acetonitrile to cyanide (generally about 2–12 hours).
Cases of acetonitrile poisoning in humans (or, to be more specific, of cyanide poisoning after exposure to acetonitrile) are rare but not unknown, by inhalation, ingestion and (possibly) by skin absorption. The symptoms, which do not usually appear for several hours after the exposure, include breathing difficulties, slow pulse rate, nausea, and vomiting. Convulsions and coma can occur in serious cases, followed by death from respiratory failure. The treatment is as for cyanide poisoning, with oxygen, sodium nitrite, and sodium thiosulfate among the most commonly used emergency treatments [5].
It has been used in formulations for nail polish remover, despite its toxicity. At least two cases have been reported of accidental poisoning of young children by acetonitrile-based nail polish remover, one of which was fatal. Acetone and ethyl acetate are often preferred as safer for domestic use, and acetonitrile has been banned in cosmetic products in the European Economic Area since March 2000.
In common with other nitriles, acetonitrile can be metabolised in microsomes, especially in the liver, to produce hydrogen cyanide, as was first shown by Pozzani et al. in 1959. The first step in this pathway is the oxidation of acetonitrile to glycolonitrile by an NADPH-dependent cytochrome P450 monooxygenase. The glycolonitrile then undergoes a spontaneous decomposition to give hydrogen cyanide and formaldehyde. Formaldehyde, a toxin and a carcinogen on its own, is further oxidized to formic acid, which is another source of toxicity.
The metabolism of acetonitrile is much slower than that of other nitriles, which accounts for its relatively low toxicity. Hence, one hour after administration of a potentially lethal dose, the concentration of cyanide in the rat brain was 1⁄20 that for a propionitrile dose 60 times lower (see table).
The relatively slow metabolism of acetonitrile to hydrogen cyanide allows more of the cyanide produced to be detoxified within the body to thiocyanate (the rhodanese pathway). It also allows more of the acetonitrile to be excreted unchanged before it is metabolised. The main pathways of excretion are by exhalation and in the urine.
Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separating tower to separate the butadiene.
It is widely used in battery applications because of its relatively high dielectric constant and ability to dissolve electrolytes. For similar reasons it is a popular solvent in cyclic voltammetry.
Its ultraviolet transparency UV cutoff, low viscosity and low chemical reactivity make it a popular choice for high-performance liquid chromatography (HPLC).
Acetonitrile plays a significant role as the dominant solvent used in the manufacture of DNA oligonucleotides from monomers.
Industrially, it is used as a solvent for the manufacture of pharmaceuticals and photographic film.
In organic synthesis, acetonitrile is a common two-carbon building block in organic synthesis of many useful chemicals, including acetamidine hydrochloride, thiamine, and α-napthaleneacetic acid. Its reaction with cyanogen chloride affords malononitrile.
Moreover, as ligand in coordination chemistry, acetonitrile is a ligand in many transition metal nitrile complexes. Being weakly basic, it is an easily displaceable ligand. For example, bis(acetonitrile)palladium dichloride is prepared by heating a suspension of palladium chloride in acetonitrile: PdCl2 + 2CH3CN → PdCl2(CH3CN)2
A related complex is [Cu(CH3CN)4]+. The CH3CN groups in these complexes are rapidly displaced by many other ligands [1].
References
[1] https://en.wikipedia.org/wiki/Acetonitrile
[2] Karakida, Ken'ichi; Fukuyama, Tsutomu; Kuchitsu, Kozo (1974). "Molecular Structures of Hydrogen Cyanide and Acetonitrile as Studied by Gas Electron Diffraction". Bulletin of the Chemical Society of Japan. 47 (2): 299–304. doi:10.1246/bcsj.47.299.
[3] Rhimi, B.; Mhamdi, M.; Ghorbel, A.; Narayana Kalevaru, V.; Martin, A.; Perez-Cadenas, M.; Guerrero-Ruiz, A. (15 May 2016). "Ammoxidation of ethylene to acetonitrile over vanadium and molybdenum supported zeolite catalysts prepared by solid-state ion exchange". Journal of Molecular Catalysis A: Chemical. 416: 127–139. doi:10.1016/j.molcata.2016.02.028.
[4] Lowe, Derek (2009). "The Great Acetonitrile Shortage". Science Translational Medicine.
[5] International Programme on Chemical Safety (1993), Environmental Health Criteria 154. Acetonitrile, Geneva: World Health Organization
You may like
Related articles And Qustion
See also
Lastest Price from Acetonitrile manufacturers

US $0.00/Kg/Drum2024-04-23
- CAS:
- 75-05-8
- Min. Order:
- 1KG
- Purity:
- 99.9%
- Supply Ability:
- 5000mt

US $10.00-30.00/kg2024-04-02
- CAS:
- 75-05-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100ton/ month